Abstract
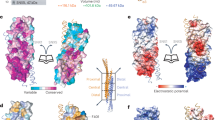
GGAs are critical for trafficking soluble proteins from the trans-Golgi network (TGN) to endosomes/lysosomes through interactions with TGN-sorting receptors, ADP-ribosylation factor (ARF) and clathrin. ARF–GTP bound to TGN membranes recruits its effector GGA by binding to the GAT domain, thus facilitating recognition of GGA for cargo-loaded receptors. Here we report the X-ray crystal structures of the human GGA1-GAT domain and the complex between ARF1–GTP and the N-terminal region of the GAT domain. When unbound, the GAT domain forms an elongated bundle of three a-helices with a hydrophobic core. Structurally, this domain, combined with the preceding VHS domain, resembles CALM, an AP180 homolog involved in endocytosis. In the complex with ARF1–GTP, a helix-loop-helix of the N-terminal part of GGA1-GAT interacts with the switches 1 and 2 of ARF1 predominantly in a hydrophobic manner. These data reveal a molecular mechanism underlying membrane recruitment of adaptor proteins by ARF–GTP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Robinson, M.S. & Bonifacino, J.S. Adaptor-related proteins. Curr. Opin. Cell Biol. 13, 444–453 (2001).
Boman, A.L., Zhang, C.-J., Zhu, X. & Kahn, R.A. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol. Biol. Cell 11, 1241–1255 (2000).
Dell'Angelica, E.C. et al. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol. 149, 81–93 (2000).
Hirst, J., Lui, W.W., Bright, N.A., Totty, N., Seaman, M.N. & Robinson, M.S. A family of proteins with γ-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol. 149, 67–79 (2000).
Poussu, A., Lohi, O. & Lehto, V.-P. Vear, a novel Golgi-associated protein with VHS and γ-adaptin “ear” domains. J. Biol. Chem. 275, 7176–7183 (2000).
Takatsu, H., Yoshino, K. & Nakayama, K. Adaptor γ ear homology domain conserved in γ-adaptin and GGA proteins that interact with γ-synergin. Biochem. Biophys. Res. Commun. 271, 719–725 (2000).
Nielsen, M.S. et al. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 20, 2180–2190 (2001).
Puertollano, R., Aguilar, R.C., Gorshkova, I., Crouch, R.J. & Bonifacino, J.S. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science 292, 1712–1716 (2001).
Zhu, Y., Doray, B., Poussu, A., Lehto, V.-P. & Kornfeld, S. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science 292, 1716–1718 (2001).
Takatsu, H., Katoh, Y., Shiba, Y. & Nakayama, K. Golgi-localizing, γ-adaptin ear homology domain, ADP-ribosylation factor-binding (GGA) proteins interact with acidic dileucine sequences within the cytoplasmic domains of sorting receptors through their Vps27p/Hrs/STAM (VHS) domains. J. Biol. Chem. 276, 28541–28545 (2001).
Doray, B., Ghosh, P., Griffith, J., Geuze, H.J. & Kornfeld, S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science 297, 1700–1703 (2002).
Zhdankina, O., Strand, N.L., Redmond, J.M. & Boman, A.L. Yeast GGA proteins interact with GTP-bound Arf and facilitate transport through the Golgi. Yeast 18, 1–18 (2001).
Puertollano, R., Randazzo, P.A., Presley, J.F., Hartnell, L.M. & Bonifacino, J.S. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell 105, 93–102 (2001).
Takatsu, H., Yoshino, K., Toda, K. & Nakayama, K. GGA proteins associate with Golgi membranes through interaction between their GGAH domains and ADP-ribosylation factors. Biochem. J. 365, 369–378 (2002).
Ford, M.G. et al. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291, 1051–1055 (2001).
Mao, Y., Chen, J., Maynard, J.A., Zhang, B. & Quiocho, F.A. A novel all helix fold of the AP180 amino-terminal domain for phosphoinositide binding and clathrin assembly in the synaptic vesicle endocytosis. Cell 104, 433–440 (2001).
Misra, S., Puertollano, R., Kato, Y., Bonifacino, J.S. & Hurley, J.H. Structural basis for acidic-cluster-dileucine sorting-signal recognition by VHS domains. Nature 415, 933–937 (2002).
Shiba, T. et al. Structural basis for recognition of acidic-cluster dileucine sequence by GGA1. Nature 415, 937–941 (2002).
Kuai, J., Boman, A.L., Arnold, R.S., Zhu, X. & Kahn, R.A. Effects of activated ADP-ribosylation factors on Golgi morphology require neither activation of phospholipase D1 nor recruitment of coatomer. J. Biol. Chem. 275, 4022–4032 (2000).
Goldberg, J. Structural and function analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell 96, 893–902 (1999).
Goldberg, J. Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell 95, 237–248 (1998).
Jacques, K.M. et al. Arf1 dissociates from the clathrin adaptor GGA prior to being inactivated by Arf GTPase-activating proteins. J. Biol. Chem. 277, 47235–47241 (2002).
Hanzal-Bayer, M., Renault, L., Roversi, P., Wittinghofer, A. & Hillig, R.C. The complex of Arl2-GTP and PDEδ: from structure to function. EMBO J. 21, 2095–2106 (2002).
Doray, B., Bruns, K., Ghosh, P. & Kornfeld, S. Autoinhibition of the ligand-binding site of GGA1/3 VHS domains by an internal acidic cluster-dileucine motif. Proc. Natl. Acad. Sci. 99, 8072–8077 (2002).
Collins, B.M., Watson, P.J. & Owen, D.J. The structure of the GGA1-GAT domain reveals the molecular basis for ARF binding and membrane association of GGAs. Dev. Cell 4, 321–332 (2003).
Leslie, A.W.G. Joint CCP4 and ESF-EACMB Newsletter on Protein Crystallography Vol. 26 (Daresbury Laboratory, Warrington, 1992).
Evans, P.R. Proceedings of the CCP4 Study Weekend on Data Collection & Processing (eds. Sawyer, L., Isaacs, N. & Bailey, S.) 114–122 (Daresbury Laboratory, Warrington; 1993).
Terwilliger, T.C. & Berendzen, J. Automated MAD and MIR structure solution. Acta Crystallogr. D 55, 849–861 (1999).
Terwilliger, T.C. Maximum-likelihood density modification. Acta Crystallogr. D 56, 965–972 (2000).
Perrakis, A., Morris, R. & Lamzin, V.S. Automated protein model building combined with iterative structure refinement. Nature Struct. Biol. 6, 458–463 (1999).
Brunger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997).
Navaza, J., AMoRE — an automated package for molecular replacement. Acta Crystallogr. A 50, 157–163 (1994).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Merritt, E.A. & Bacon, D.J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505–524 (1997).
Lawrence, M.C. & Bourke, P. CONSCRIPT: a program for generating electron density isosurfaces for presentation in protein crystallography. J. Appl. Crystallogr. 33, 990–991 (2000).
Sreerama, N. & Woody, R.W. A self-consistent method for the analysis of protein secondary structure from circular dichroism. Anal. Biochem. 209, 32–44 (1993).
Acknowledgements
Coordinates of the two complex structures, ARF–ARFGAP and ARF–Sec7 domain, were provided by J. Goldberg. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, from the Japan Society for Promotion of Science (fellowship to H.T.), from the University of Tsukuba Research Projects, and by Protein 3000 Project of the MEXT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Shiba, T., Kawasaki, M., Takatsu, H. et al. Molecular mechanism of membrane recruitment of GGA by ARF in lysosomal protein transport. Nat Struct Mol Biol 10, 386–393 (2003). https://doi.org/10.1038/nsb920
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb920
This article is cited by
-
Regulation of α2B-Adrenergic Receptor Cell Surface Transport by GGA1 and GGA2
Scientific Reports (2016)
-
Application of Gaussia luciferase in bicistronic and non-conventional secretion reporter constructs
BMC Biochemistry (2014)
-
Structural basis for membrane targeting of the BBSome by ARL6
Nature Structural & Molecular Biology (2014)
-
Adaptor proteins in protein trafficking between endomembrane compartments in plants
Journal of Plant Biology (2014)
-
Structural basis for Arf6-MKLP1 complex formation on the Flemming body responsible for cytokinesis
The EMBO Journal (2012)