Abstract
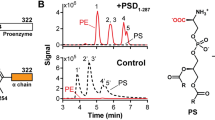
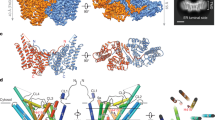
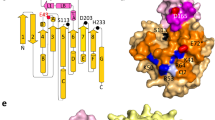
Enzymes in lipid metabolism acquire and deliver hydrophobic substrates and products from within lipid bilayers. The structure at 2.55 Å of one isozyme of a constitutive enzyme in lipid A biosynthesis, LpxI from Caulobacter crescentus, has a novel fold. Two domains close around a completely sequestered substrate, UDP-2,3-diacylglucosamine, and open to release products either to the neighboring enzyme in a putative multienzyme complex or to the bilayer. Mutation analysis identifies Asp225 as key to Mg2+-catalyzed diphosphate hydrolysis. These structures provide snapshots of the enzymatic synthesis of a critical lipid A precursor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Raetz, C.R.H. & Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700 (2002).
Raetz, C.R.H., Reynolds, C.M., Trent, M.S. & Bishop, R.E. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 76, 295–329 (2007).
Croxen, M.A. & Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 8, 26–38 (2010).
Miller, S.I., Ernst, R.K. & Bader, M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 3, 36–46 (2005).
Babinski, K.J., Kanjilal, S.J. & Raetz, C.R.H. Accumulation of the lipid A precursor UDP-2,3-diacylglucosamine in an Escherichia coli mutant lacking the lpxH gene. J. Biol. Chem. 277, 25947–25956 (2002).
Babinski, K.J. & Raetz, C.R.H. Identification of a gene encoding a novel Escherichia coli UDP-2,3-diacylglucosamine hydrolase. FASEB J. 12, A1288 (1998).
Metzger, L.E. & Raetz, C.R. An alternative route for UDP-diacylglucosamine hydrolysis in bacterial lipid A biosynthesis. Biochemistry 49, 6715–6726 (2010).
Stahelin, R.V. Lipid binding domains: more than simple lipid effectors. J. Lipid Res. 50, S299–S304 (2009).
Ko, T.P. et al. Crystal structure of yeast cytosine deaminase. Insights into enzyme mechanism and evolution. J. Biol. Chem. 278, 19111–19117 (2003).
Anderson, M.S., Bulawa, C.E. & Raetz, C.R.H. The biosynthesis of gram-negative endotoxin: formation of lipid A precursors from UDP-GlcNAc in extracts of Escherichia coli. J. Biol. Chem. 260, 15536–15541 (1985).
Huang, B. MetaPocket: a meta approach to improve protein ligand binding site prediction. OMICS 13, 325–330 (2009).
Coleman, R.G. & Sharp, K.A. Protein pockets: inventory, shape, and comparison. J. Chem. Inf. Model. 50, 589–603 (2010).
Bligh, E.G. & Dyer, J.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Garrett, T.A., Kordestani, R. & Raetz, C.R.H. Quantification of cardiolipin by liquid chromatography-electrospray ionization mass spectrometry. Methods Enzymol 433, 213–230 (2007).
Metzger, L.E. & Raetz, C.R.H. Purification and characterization of the lipid A disaccharide synthase (LpxB) from Escherichia coli, a peripheral membrane protein. Biochemistry 48, 11559–11571 (2009).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Schaaf, G. et al. Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the Sec14 superfamily. Mol. Cell 29, 191–206 (2008).
Epand, R.M. & Epand, R.F. Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochimica Et Biophysica Acta 1788, 289–294 (2009).
Silk, J.D., Salio, M., Brown, J., Jones, E.Y. & Cerundolo, V. Structural and functional aspects of lipid binding by CD1 molecules. Annu. Rev. Cell Dev. Biol. 24, 369–395 (2008).
Wang, J. et al. Lipid binding orientation within CD1d affects recognition of Borrelia burgorferi antigens by NKT cells. Proc. Natl. Acad. Sci. USA 107, 1535–1540 (2010).
Park, B.S. et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458, 1191–1195 (2009).
Bryant, C.E., Spring, D.R., Gangloff, M. & Gay, N.J. The molecular basis of the host response to lipopolysaccharide. Nat. Rev. Microbiol. 8, 8–14 (2010).
Williams, A.H. & Raetz, C.R.H. Structural basis for the acyl chain selectivity and mechanism of UDP-N-acetylglucosamine acyltransferase. Proc. Natl. Acad. Sci. USA 104, 13543–13550 (2007).
Robins, L.I., Williams, A.H. & Raetz, C.R.H. Structural basis for the sugar nucleotide and acyl-chain selectivity of Leptospira interrogans LpxA. Biochemistry 48, 6191–6201 (2009).
Lemmon, M.A. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 9, 99–111 (2008).
Kutateladze, T.G. Mechanistic similarities in docking of the FYVE and PX domains to phosphatidylinositol 3-phosphate containing membranes. Prog. Lipid Res. 46, 315–327 (2007).
Bessman, M.J., Frick, D.N. & O'Handley, S.F. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J. Biol. Chem. 271, 25059–25062 (1996).
Gabelli, S.B. et al. Structure and mechanism of GDP-mannose glycosyl hydrolase, a nudix enzyme that cleaves at carbon instead of phosphorus. Structure 12, 927–935 (2004).
Gahan, L.R., Smith, S.J., Neves, A. & Schenk, G. Phosphate ester hydrolysis: metal complexes as purple acid phosphatase and phosphotriesterase analogues. Eur. J. Inorg. Chem 2009, 2745–2758 (2009).
Kraszewska, E. The plant Nudix hydrolase family. Acta Biochim. Pol. 55, 663–671 (2008).
McLennan, A.G. The Nudix hydrolase superfamily. Cell. Mol. Life Sci. 63, 123–143 (2006).
Lairson, L.L., Henrissat, B., Davies, G.J. & Withers, S.G. Glycosyltransferases: structures, functions, and mechanisms. Annu. Rev. Biochem. 77, 521–555 (2008).
Anderson, M.S., Robertson, A.D., Macher, I. & Raetz, C.R.H. Biosynthesis of lipid A in Escherichia coli: identification of UDP-3-O-(R-3-hydroxymyristoyl)-α-D-glucosamine as a precursor of UDP-N2-O3-bis-(R-3-hydroxymyristoyl)-α- D-glucosamine. Biochemistry 27, 1908–1917 (1988).
Kelly, T.M., Stachula, S.A., Raetz, C.R.H. & Anderson, M.S. The firA gene of Escherichia coli encodes UDP-3-O-(R-3-hydroxymyristoyl)-α- D-glucosamine N-acyltransferase: the third step of endotoxin biosynthesis. J. Biol. Chem. 268, 19866–19874 (1993).
Miroux, B. & Walker, J.E. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260, 289–298 (1996).
Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. HKL-3000: the integration of data reduction and structure solution—from diffraction images to an initial model in minutes. Acta Crystallogr. D Biol. Crystallogr. 62, 859–866 (2006).
Holton, J. & Alber, T. Automated protein crystal structure determination using ELVES. Proc. Natl. Acad. Sci. USA 101, 1537–1542 (2004).
Adams, P.D. et al. The Phenix software for automated determination of macromolecular structures. Methods 55, 94–106 (2011).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Krissinel, E.B. et al. The new CCP4 Coordinate Library as a toolkit for the design of coordinate-related applications in protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 60, 2250–2255 (2004).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Headd, J.J. et al. Autofix for backward-fit sidechains: using MolProbity and real-space refinement to put misfits in their place. J. Struct. Funct. Genomics 10, 83–93 (2009).
Davis, I.W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
Davis, I.W., Murray, L.W., Richardson, J.S. & Richardson, D.C. MOLPROBITY: structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic Acids Res. 32, W615–W619 (2004).
Garrett, T.A. & Raetz, C.R.H. Identification of new lipid metabolites in Escherichia coli total lipid extracts using electrospray ionization quadrupole time of flight mass spectrometry. FASEB J. 21, A605 (2007).
Laue, T.M. & Stafford, W.F. III. Modern applications of analytical ultracentrifugation. Annu. Rev. Biophys. Biomol. Struct. 28, 75–100 (1999).
Lebowitz, J., Lewis, M.S. & Schuck, P. Modern analytical ultracentrifugation in protein science: a tutorial review. Protein Sci. 11, 2067–2079 (2002).
Schuck, P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal. Biochem. 320, 104–124 (2003).
Acknowledgements
We dedicate this paper to the memory of coauthor Christian R.H. Raetz (1946–2011), our dear colleague and friend. We thank H.S. Chung, D.A. Six, Z. Guan, G. Laird, J.M. Holton, N.I. Nicely, J.E. Pak and J.W. Werner-Allen for helpful discussions. We thank H.J. Sage for analytical ultracentrifugation services. This research was supported by the US National Institutes of Health grants U54GM094625 (to R.M.S.), GM24485 (to R.M.S.), GM51310 (to C.R.H.R.) and GM069338 (to C.R.H.R.).
Author information
Authors and Affiliations
Contributions
L.E.M. purified and biochemically characterized the protein. L.E.M. and J.K.L. determined and optimized the crystallization conditions, collected the crystallographic data and solved the structures. L.E.M., J.K.L. and J.S.F.-M. refined the structures. L.E.M., J.S.F.-M., C.R.H.R. and R.M.S. analyzed and interpreted the structures. L.E.M. performed and analyzed, with C.R.H.R., the LC/MS experiments. L.E.M. wrote the manuscript and J.S.F.-M. and R.M.S. edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 1 (PDF 1549 kb)
Rights and permissions
About this article
Cite this article
Metzger, L., Lee, J., Finer-Moore, J. et al. LpxI structures reveal how a lipid A precursor is synthesized. Nat Struct Mol Biol 19, 1132–1138 (2012). https://doi.org/10.1038/nsmb.2393
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2393
This article is cited by
-
Overcoming Klebsiella pneumoniae antibiotic resistance: new insights into mechanisms and drug discovery
Beni-Suef University Journal of Basic and Applied Sciences (2024)
-
Crystal structure of lipid A disaccharide synthase LpxB from Escherichia coli
Nature Communications (2018)
-
Crystal structures of the UDP-diacylglucosamine pyrophosphohydrase LpxH from Pseudomonas aeruginosa
Scientific Reports (2016)
-
Structure of the essential Haemophilus influenzae UDP-diacylglucosamine pyrophosphohydrolase LpxH in lipid A biosynthesis
Nature Microbiology (2016)