Abstract
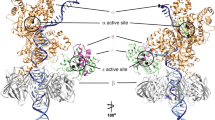
All DNA replicases achieve high fidelity by a conserved mechanism, but each translesion polymerase carries out mutagenic DNA synthesis in its own way. Here we report crystal structures of human DNA polymerase ν (Pol ν), which is homologous to high-fidelity replicases yet is error prone. Instead of a simple open-to-closed movement of the O helix upon binding of a correct incoming nucleotide, Pol ν has a different open state and requires the finger domain to swing sideways and undergo both opening and closing motions to accommodate the nascent base pair. A single–amino acid substitution in the O helix of the finger domain improves the fidelity of Pol ν nearly ten-fold. A unique cavity and the flexibility of the thumb domain allow Pol ν to generate and accommodate a looped-out primer strand. Primer loop-out may be a mechanism for DNA trinucloetide-repeat expansion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Takata, K., Shimizu, T., Iwai, S. & Wood, R.D. Human DNA polymerase N (POLN) is a low fidelity enzyme capable of error-free bypass of 5S-thymine glycol. J. Biol. Chem. 281, 23445–23455 (2006).
Arana, M.E., Takata, K., Garcia-Diaz, M., Wood, R.D. & Kunkel, T.A. A unique error signature for human DNA polymerase nu. DNA Repair (Amst.) 6, 213–223 (2007).
Arana, M.E., Seki, M., Wood, R.D., Rogozin, I.B. & Kunkel, T.A. Low-fidelity DNA synthesis by human DNA polymerase theta. Nucleic Acids Res. 36, 3847–3856 (2008).
Seki, M. & Wood, R.D. DNA polymerase theta (POLQ) can extend from mismatches and from bases opposite a (6–4) photoproduct. DNA Repair (Amst.) 7, 119–127 (2008).
Takata, K., Arana, M.E., Seki, M., Kunkel, T.A. & Wood, R.D. Evolutionary conservation of residues in vertebrate DNA polymerase N conferring low fidelity and bypass activity. Nucleic Acids Res. 38, 3233–3244 (2010).
Kohzaki, M. et al. DNA polymerases nu and theta are required for efficient immunoglobulin V gene diversification in chicken. J. Cell Biol. 189, 1117–1127 (2010).
Yousefzadeh, M.J. & Wood, R.D. DNA polymerase POLQ and cellular defense against DNA damage. DNA Repair (Amst.) 12, 1–9 (2013).
Koole, W. et al. A polymerase theta-dependent repair pathway suppresses extensive genomic instability at endogenous G4 DNA sites. Nat. Commun. 5, 3216 (2014).
Esposito, G. et al. Disruption of the Rev3l-encoded catalytic subunit of polymerase zeta in mice results in early embryonic lethality. Curr. Biol. 10, 1221–1224 (2000).
Lange, S.S. et al. Dual role for mammalian DNA polymerase zeta in maintaining genome stability and proliferative responses. Proc. Natl. Acad. Sci. USA 110, E687–E696 (2013).
Wood, R.D. DNA damage tolerance and a web of connections with DNA repair at Yale. Yale J. Biol. Med. 86, 507–516 (2013).
Reha-Krantz, L.J. DNA polymerase proofreading: multiple roles maintain genome stability. Biochim. Biophys. Acta 1804, 1049–1063 (2010).
Swan, M.K., Johnson, R.E., Prakash, L., Prakash, S. & Aggarwal, A.K. Structural basis of high-fidelity DNA synthesis by yeast DNA polymerase δ. Nat. Struct. Mol. Biol. 16, 979–986 (2009).
Hogg, M. et al. Structural basis for processive DNA synthesis by yeast DNA polymerase ɛ. Nat. Struct. Mol. Biol. 21, 49–55 (2014).
Lee, Y.S., Kennedy, W.D. & Yin, Y.W. Structural insight into processive human mitochondrial DNA synthesis and disease-related polymerase mutations. Cell 139, 312–324 (2009).
Doublié, S., Sawaya, M.R. & Ellenberger, T. An open and closed case for all polymerases. Structure 7, R31–R35 (1999).
Xia, S. & Konigsberg, W.H. RB69 DNA polymerase structure, kinetics, and fidelity. Biochemistry 53, 2752–2767 (2014).
Wang, F. & Yang, W. Structural insight into translesion synthesis by DNA Pol II. Cell 139, 1279–1289 (2009).
Yang, W. & Woodgate, R. What a difference a decade makes: insights into translesion DNA synthesis. Proc. Natl. Acad. Sci. USA 104, 15591–15598 (2007).
Seki, M., Marini, F. & Wood, R.D. POLQ (Pol theta), a DNA polymerase and DNA-dependent ATPase in human cells. Nucleic Acids Res. 31, 6117–6126 (2003).
Lee, Y.S., Gregory, M.T. & Yang, W. Human Pol zeta purified with accessory subunits is active in translesion DNA synthesis and complements Pol eta in cisplatin bypass. Proc. Natl. Acad. Sci. USA 111, 2954–2959 (2014).
Marini, F., Kim, N., Schuffert, A. & Wood, R.D. POLN, a nuclear PolA family DNA polymerase homologous to the DNA cross-link sensitivity protein Mus308. J. Biol. Chem. 278, 32014–32019 (2003).
Sharief, F.S., Vojta, P.J., Ropp, P.A. & Copeland, W.C. Cloning and chromosomal mapping of the human DNA polymerase theta (POLQ), the eighth human DNA polymerase. Genomics 59, 90–96 (1999).
Seki, M. et al. High-efficiency bypass of DNA damage by human DNA polymerase Q. EMBO J. 23, 4484–4494 (2004).
Hogg, M., Seki, M., Wood, R.D., Doublie, S. & Wallace, S.S. Lesion bypass activity of DNA polymerase theta (POLQ) is an intrinsic property of the pol domain and depends on unique sequence inserts. J. Mol. Biol. 405, 642–652 (2011).
Arana, M.E., Potapova, O., Kunkel, T.A. & Joyce, C.M. Kinetic analysis of the unique error signature of human DNA polymerase nu. Biochemistry 50, 10126–10135 (2011).
Arana, M.E., Powell, G.K., Edwards, L.L., Kunkel, T.A. & Petrovich, R.M. Refolding active human DNA polymerase nu from inclusion bodies. Protein Expr. Purif. 70, 163–171 (2010).
Evans, R.J. et al. Structure of PolC reveals unique DNA binding and fidelity determinants. Proc. Natl. Acad. Sci. USA 105, 20695–20700 (2008).
Moon, A.F. et al. The X family portrait: structural insights into biological functions of X family polymerases. DNA Repair (Amst.) 6, 1709–1725 (2007).
Garcia-Diaz, M., Bebenek, K., Krahn, J.M., Kunkel, T.A. & Pedersen, L.C. A closed conformation for the Pol λ catalytic cycle. Nat. Struct. Mol. Biol. 12, 97–98 (2005).
Zhao, Y. et al. Structural basis of human DNA polymerase eta-mediated chemoresistance to cisplatin. Proc. Natl. Acad. Sci. USA 109, 7269–7274 (2012).
Li, Y., Korolev, S. & Waksman, G. Crystal structures of open and closed forms of binary and ternary complexes of the large fragment of Thermus aquaticus DNA polymerase I: structural basis for nucleotide incorporation. EMBO J. 17, 7514–7525 (1998).
Wang, W., Hellinga, H.W. & Beese, L.S. Structural evidence for the rare tautomer hypothesis of spontaneous mutagenesis. Proc. Natl. Acad. Sci. USA 108, 17644–17648 (2011).
Johnson, S.J., Taylor, J.S. & Beese, L.S. Processive DNA synthesis observed in a polymerase crystal suggests a mechanism for the prevention of frameshift mutations. Proc. Natl. Acad. Sci. USA 100, 3895–3900 (2003).
Wu, E.Y. & Beese, L.S. The structure of a high fidelity DNA polymerase bound to a mismatched nucleotide reveals an “ajar” intermediate conformation in the nucleotide selection mechanism. J. Biol. Chem. 286, 19758–19767 (2011).
Zhao, Y. et al. Mechanism of somatic hypermutation at the WA motif by human DNA polymerase eta. Proc. Natl. Acad. Sci. USA 110, 8146–8151 (2013).
Brueckner, F., Ortiz, J. & Cramer, P. A movie of the RNA polymerase nucleotide addition cycle. Curr. Opin. Struct. Biol. 19, 294–299 (2009).
Steitz, T.A. Visualizing polynucleotide polymerase machines at work. EMBO J. 25, 3458–3468 (2006).
Yang, W. An overview of Y-family DNA polymerases and a case study of human DNA polymerase eta. Biochemistry 53, 2793–2803 (2014).
Yin, Y.W. & Steitz, T.A. Structural basis for the transition from initiation to elongation transcription in T7 RNA polymerase. Science 298, 1387–1395 (2002).
Cleary, J.D., Nichol, K., Wang, Y.H. & Pearson, C.E. Evidence of cis-acting factors in replication-mediated trinucleotide repeat instability in primate cells. Nat. Genet. 31, 37–46 (2002).
Budworth, H. & McMurray, C.T. A brief history of triplet repeat diseases. Methods Mol. Biol. 1010, 3–17 (2013).
Mirkin, E.V. & Mirkin, S.M. To switch or not to switch: at the origin of repeat expansion disease. Mol. Cell 53, 1–3 (2014).
Gacy, A.M. et al. GAA instability in Friedreich's Ataxia shares a common, DNA-directed and intraallelic mechanism with other trinucleotide diseases. Mol. Cell 1, 583–593 (1998).
Aricescu, A.R., Lu, W. & Jones, E.Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D Biol. Crystallogr. 62, 1243–1250 (2006).
Jensen, R.B., Carreira, A. & Kowalczykowski, S.C. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 467, 678–683 (2010).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Acknowledgements
We thank D. Leahy and R. Craigie for editing the manuscript. The research was supported by the intramural research program of the US National Institutes of Health (DK036146-08, W.Y.).
Author information
Authors and Affiliations
Contributions
Y.-S.L. carried out most of the experiments. Y.G. helped with kinetic measurement of K678A-mutant Pol ν and with data deposition. Y.-S.L. and W.Y. designed the project and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Cloning and characterization of Pol ν variants.
(a) Sequence alignment of Pol ν and the crystallized Klenow fragment of Bacillus Pol I. The first residues in Pol ν77, ν76, ν75 and ν74 are highlighted in green.
(b) Diagrams of expression constructs of four Pol ν variants. The His8 N-terminal to the two copies of maltose-binding protein (MBP) is not shown.
(c) PreScission protease digestion of Pol ν77, ν76, ν75 and ν74 after one-step purification with amylose resin. The results were analyzed by SDS gel.
(d) Size-exclusion chromatography analysis showed that Pol ν77 and DNA formed a stable complex of 1:1 molar ratio.
(e) Primer extension of the DNA substrate (with sequence shown) by tag-free Pol ν77, ν76 and ν75 (10 nM each) in the presence of all four nucleotides (dNTPs, 100µM total). Reactions were carried out with 100nM DNA in 20 mM Tris (pH 8.0), 80 mM NaCl, 1mM DTT, 10 mM MgCl2 and 0.1 mg/ml BSA at 37°C for 5 min. Pol ν75 was as active as Pol ν77.
Supplementary Figure 2 Purification and activity of WT and mutant Pol ν75.
(a) Results of Pol ν75 purification were shown in SDS gel. S200 stands for Superdex 200.
(b) Nucleotide selectivity. Four DNAs (100nM each) with varying templating nucleotide (A, G, T or C) were used to assay nucleotide selection by Pol ν77 in 10 µL of 20 mM Tris (pH 7.4), 50 mM NaCl, 0.1mg/ml BSA, 1 mM DTT, 5 mM MgCl2 and 100µM of dNTP at 37°C for 10 min.
(c) Purity of WT, E675R, K679A, and ΔIns2 Pol ν75 examined by SDS gel.
(d) The catalytic activities were assayed using a normal DNA substrate (sequence shown) as described in Methods (DNA synthesis) except for 5-min reaction time (not 10-min). E675R and ΔIns2 had reduced activity.
Supplementary Figure 3 Diagram of DNA sequences used in crystallizing Pol ν75.
(a) DNA in the Ndna1 crystal. The designed and actual DNA crystallized are shown. Pol ν75 is shown as a Packman.
(b) The SAD-MR experimental electron density map is contoured at 1.0 σ in pea green. The 4 Br sites are obvious based on the anomalous peaks contoured at 8.0 σ in blue.
(c) DNAs designed for Ndna2 and Ndna3 are shown side-by-side. The 2Fo-Fc (1.0 σ) and Fo-Fc map (3.0 σ) corresponding to the DNAs are shown below. In Ndna2 the 4-nt overhang formed 5 bps overhang at a crystal dyad instead of 4 bps as in Ndna3, which was explained by a misalignment and gap-filling by dAMPNPP (a non-hydrolyzable analog of incoming nucleotide) as diagramed.
(d) DNA in Ndna4. The designed and actual DNA crystallized are shown side-by-side. The overhang at the crystal dyad also formed 5 bps as in Ndna2.
Supplementary Figure 4 Comparison of Pol ν75 and E. coli Klenow fragment on looped-out primer DNA.
(a) Sequences and structures of normal (fully base paired, Full-bp or F-bp) DNA and seven variations with looped-out nucleotide in primer.
(b) Primer extension assay of Pol ν75 (40 nM) and the E. coli Klenow fragment (New England BioLabs, 0.0357 unit) with different DNA substrates in the presence of each dNTP (A, G, T or C) or all four dNTPs (4). Reactions were carried out as described in Methods (DNA synthesis) except for pH 7.4 (not pH 8.3) and 5-min reaction time (not 10-min). Pol ν75 was active with P[3,1] and P[4 or 5,1], while Klenow had weak activity with P[4 or 5,1].
Supplementary Figure 5 Cloning, purification and activity of Pol θ90 and Pol θ86.
(a) According to the secondary structure prediction and the previously published active Pol θ90 construct, the cloning site of Pol θ86 was chosen to remove predicted additional random coils (indicated as C)
(b) Expression, purification and PreScission cleavage of 2MBP tagged Pol θ90 and Pol θ86. Results are shown in SDS gel. The 2MBP tag and tag-free Pol θ90 and Pol θ86 have similar sizes and overlap on the SDS gel.
(c) Pol θ86 was purified as Pol ν75. The elution points from Heparin and Superdex 200 (S200) of the two proteins differed.
(d) Pol θ90 and Pol θ86 were equally active in DNA synthesis, before or after PreScission cleavage.
(e) Activity comparison of Pol ν75 and Pol θ86. The specific activity of Pol ν75 is roughly one fourth of that of Pol θ86. Reactions were carried out as described in Methods (DNA synthesis).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2 (PDF 1983 kb)
Finger movement between the conventional open and closed state exemplified by bacillus Pol I
The binary complex (4BDP) and ternary complex (3THV) were compared. The three domains of the catalytic core are color-coded and the Exo domain is shown in silver. The M, N (lilac) and O (purple) helices undergo the large closing movement (40° rotation). The Oa and Ob helices move slightly towards the N and O helices. (MOV 1962 kb)
Conformational changes of Pol ν from the unusual open (Ndna3) to the closed state (Ndna2).
The Oa and Ob helices are colored in light blue. The remaining regions are colored as in movie 1. The M helix is disordered in Pol ν. The N (lilac) and O (purple) helices undergo a closing movement towards the DNA (25° rotation). The Oa and Ob helices move outwards by a 17° rotation. (MOV 1936 kb)
The 30° rotation of the thumb domain in Pol ν
The conformational changes between Ndna2 (closed finger and thumb-in) and Ndna4 (closed finger and thumb-out) structure. Y686, shown in stick-and-ball model, is a placeholder for the incoming templating base. (MOV 1736 kb)
Rights and permissions
About this article
Cite this article
Lee, YS., Gao, Y. & Yang, W. How a homolog of high-fidelity replicases conducts mutagenic DNA synthesis. Nat Struct Mol Biol 22, 298–303 (2015). https://doi.org/10.1038/nsmb.2985
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2985
This article is cited by
-
Centuries of genome instability and evolution in soft-shell clam, Mya arenaria, bivalve transmissible neoplasia
Nature Cancer (2023)
-
Quaternary structural diversity in eukaryotic DNA polymerases: monomeric to multimeric form
Current Genetics (2020)
-
Structure and function relationships in mammalian DNA polymerases
Cellular and Molecular Life Sciences (2020)
-
How DNA polymerases catalyse replication and repair with contrasting fidelity
Nature Reviews Chemistry (2017)
-
Structures of human DNA polymerases ν and θ expose their end game
Nature Structural & Molecular Biology (2015)