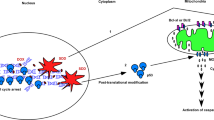
Abstract
Cells’ ability to evade cell death and to proliferate post geno-/cell-toxic stresses likely leads to formation of cancer. Activation of p38MAPK and p53 following these stresses helps protect cells against cancer development by initiating apoptosis. The duration of p38MAPK and p53 activation is regulated by the WIP1 phosphatase. BRCA1-IRIS triggers WIP1 expression in a p53-dependent and -independent manner. BRCA1-IRIS triggers the expression and cytoplasmic localization of the mRNA stabilization and translation inducer, HuR, that binds p53 and PPM1D mRNA. Hence, BRCA1-IRIS overexpression inactivates p38MAPK and/or p53 by upregulating WIP1 expression. BRCA1-IRIS abrogation of the homeostatic balance maintained by the p38MAPK–p53–WIP1 pathway suppressed cell death induced by a lethal dose of short-wavelength UV light, and high dosage of etoposide or H2O2, and allowed cells to survive and proliferate post geno-/cell-toxic stresses. This mechanism represents a new link between geno-/cell-toxic stress and aggressive breast cancer formation in p53 wild-type cells.
Similar content being viewed by others
Introduction
The tumor suppressor p53 is activated in response to a variety of mitogenic and stressful stimuli (Moll and Schramm, 1998; Hammond and Giaccia, 2005; Latonen and Laiho, 2005). Following genotoxic stresses, p53 expression increases primarily through modulation of the steady-state level of the protein through phosphorylation and acetylation (Shieh et al., 1997; Dumaz and Meek, 1999; Sykes et al., 2009). Stabilized p53 induces transcription of many cell cycle arrest and apoptosis genes, such as Gadd45 and p21WAF1, Bcl-2, Bax, PUMA and MDM2 (Moll and Schramm, 1998; Hammond and Giaccia, 2005; Latonen and Laiho, 2005). Moreover, the stabilizing factor, HuR, binds and stabilizes p53 mRNA following ultraviolet irradiation (Yao et al., 1993; Mazan-Mamczarz et al., 2003; Tong and Pelling, 2009). HuR is a nucleocytoplasmic shuttling protein that binds to the AU-rich element in the 3′ untranslated region of mRNAs of many genes, such as VEGF, p21, Cyclin A and B1, c-fos and p27 (Al-Mohanna et al., 2007; Sakuma et al., 2008).
Protein phosphatase magnesium-dependent 1 delta (PPM1D), the gene encoding for the serine/threonine phosphatase WIP1 (for wild-type p53-induced phosphatase 1), is located on human chromosome 17q23–24 (Fiscella et al., 1997). PPM1D amplification and overexpression is associated with poor clinical outcomes in neuroblastoma, breast and ovarian clear cell carcinomas (Hirasawa et al., 2003; Saito-Ohara et al., 2003; Bulavin et al., 2004). WIP1 is expressed under various stress conditions, such as infra-red and ultraviolet, in a p53-dependent manner, where it inactivates p38MAPK and p53 itself (Takekawa et al., 2000; Yamaguchi et al., 2005).
p38MAPK is a serine/threonine kinase, and is a member of the mitogen-activated protein kinase (MAPK) family that includes the extracellular signal-regulated protein kinases and the stress-activated c-Jun N-terminal kinases (Sturgill, 2008). Following geno-/cell-toxic stresses, p38MAPK is activated by phosphorylation of threonine 180/tyrosine 182 (T180/Y182) (Fornace, 1999; Sturgill, 2008). Activated p38MAPK then activates p53 by phosphorylating its serine 15, 33 and 46, thereby inducing cell cycle arrest or apoptosis (Fornace, 1999).
Despite its original discovery as a product of the tumor suppressor BRCA1 locus, and despite sharing 1365 from its 1399 residues with the tumor suppressor protein product of this locus, BRCA1/p220, BRCA1-IRIS has oncogenic properties. BRCA1-IRIS promotes DNA replication during the S phase, in part by suppressing the inhibitory function of the DNA replication suppressor, Geminin (ElShamy and Livingston, 2004), and cell proliferation by upregulating the Cyclin D1 expression, directly by binding to its promoter through c-Jun/AP1 transcription complex and activates its transcription (Nakuci et al., 2006), or indirectly by suppressing the expression of the c-Jun N-terminal kinase-specific inactivating phosphatase DUSP3/VHR (Hao and ElShamy, 2007).
Here, we examined the mechanisms governing the production of breast cancer in wild-type p53 cells. Short-wavelength UV light (UVC), etoposide or H2O2 exposure triggered BRCA1-IRIS expression in normal and breast cancer cell lines, which triggered WIP1 expression in a p53-dependent and -independent manner. Independently, BRCA1-IRIS promoted the expression and cytoplasmic localization of HuR that binds p53 and PPM1D mRNAs and enhanced their stability and translation. Accordingly, BRCA1-IRIS silencing induced apoptosis in breast cancer cells exposed to UVC, etoposide or H2O2, and p53 silencing or p38MAPK inactivation augmented that apoptosis. However, BRCA1-IRIS overexpression in normal human mammary epithelial (HME) cells suppressed cell death induced by UVC, etoposide or H2O2, and these damaged cells continued to proliferate, especially when p53 was silenced or p38MAPK was inactivated in them. We propose that this mechanism accounts, at least in part, for the initiation of breast cancer in patients with the wild-type p53 gene.
Results
BRCA1-IRIS triggers WIP1 expression in a p53-dependent and -independent manner
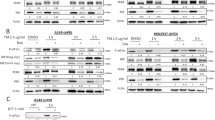
Recently we showed that silencing of BRCA1-IRIS activates p38MAPK (Hao and ElShamy, 2007). To test whether alterations in WIP1 expression is responsible for that effect (Hickson et al., 2007), BT474, MCF7 and SKBR3 breast cancer cell lines endogenously overexpressing BRCA1-IRIS (Figure 1a) were transfected with luciferase (hereafter control) or BRCA1-IRIS small interfering RNAs (siRNAs). BRCA1-IRIS silencing significantly reduced WIP1 mRNA (Figure 1b) and protein (Figure 1d) levels in all the cell lines, leading to a significant increase in p-p38MAPK with no effect on the total p38MAPK level (Figure 1d). BRCA1-IRIS silencing also reduced p53 mRNA (Figure 1b) and total and phosphorylated protein (detected using a mix of p-S15/37/46-p53 antibodies (hereafter p-p53), Figure 1d) levels, consistent with WIP1 being the p53 target.
p53-dependent and -independent induction of WIP1 by BRCA1-IRIS. (a) Expression of BRCA1-IRIS in the breast cancer cell lines BT474 and SKBR3 with p53-mutant or MCF7 with wild-type p53 and HME 2 doxycycline inducible His-BRCA1-IRIS, IRIS2 and IRIS3 and uninducible, IRIS1. Expression of BRCA1-IRIS, p53, PPM1D and GAPDH mRNAs in BT474-, MCF7- and SKBR3-silenced cells (b), or HME, IRIS1 or induced IRIS2 and IRIS3 (c). Expression of the indicated proteins in BRCA1-IRIS-silenced BT474, MCF7 and SKBR3 (d) or HME, IRIS1 and induced IRIS2 and IRIS3 (e). (f) Expression of WIP after BRCA1-IRIS silencing in MCF7 cells stably expressing shp53, HEK293T or following infection of SAOS2 cells with His-tagged BRCA1-IRIS cDNA. (g) Transactivation assay in HME cells co-transfected with pGL3-hp53, pGL3-hPPM1D1 or pGL3-hCycD1 promoters and vehicle (none), siGFP, siIRIS, pcDNA3 or pcDNA3-IRIS or pcDNA-p53 or pcDNA-WIP1 as indicated.
Three HME cell lines, one carrying uninducible (IRIS1) and two carrying inducible (IRIS2 and IRIS3) His-tagged BRCA1-IRIS alleles, were generated. IRIS2 and IRIS3 express three- to four-fold more BRCA1-IRIS compared with control cells when induced with 2 μg/ml of doxycycline for 72 h (Figure 1a). BRCA1-IRIS overexpression increased p53 and WIP1 mRNA (Figure 1c) and protein (Figure 1e) levels compared with control cells, and had no effect on the total p38MAPK protein level (Figure 1e). BRCA1-IRIS overexpression increased p-p53 (detected using a mix of p-S15/37/46-p53 antibodies) level perhaps by an oncogene-induced cellular stress-related mechanism (Lowe, 1999). To identify the exact site on p53 that is phosphorylated in BRCA1-IRIS overexpressing cells, HME and induced IRIS2 and IRIS3 total cell extracts were probed with anti-p-S15, S37 or S46-p53 antibodies, separately. BRCA1-IRIS overexpression triggered phosphorylation of all three sites on p53 (Supplementary Figure 1a). No p-p38MAPK was observed in any of the cell lines (Figure 1e), for which an explanation is given in the discussion.
BRCA1-IRIS silencing reduced WIP1 expression in p53 wild-type, MCF-7 and p53 mutant, BT474 and SKBR3 cells. Does BRCA1-IRIS induce WIP1 in a p53-independent manner? BRCA1-IRIS was silenced in MCF7 cells stably expressing p53 small interfering hairpin. Compared with control MCF7 cells, these cells expressed equal but ∼30% reduced BRCA1-IRIS and WIP1, respectively (Figure 1f). BRCA1-IRIS silencing decreased WIP1 levels in both cell lines (Figure 1f). BRCA1-IRIS silencing in human embryonic kidney cells infected with SV40 virus (HEK293T), in which SV40 large T antigen binds and inactivates p53 (based on low p21 expression, data not shown) also suppressed WIP1 expression (Figure 1f). Finally, when the p53-negative osteosarcoma cell line SAOS2 was infected with His-tagged BRCA1-IRIS expressing vector an increase in WIP1 level compared with vector expressing cells was observed (Figure 1f in keeping with p53-indepedent induction of WIP1 by BRCA1-IRIS.
BRCA1-IRIS triggers p53 and WIP1 expression in a transcriptional-independent manner
Whether BRCA1-IRIS binds and activates p53 and PPM1D promoters was studied next. Human Cyclin D1 (positive control, see Nakuci et al., 2006), p53 and PPM1D promoter regions were cloned upstream of the luciferase gene in the pGL3-Basic vector to produce pGL3-hCycD1 (a 2.5 kb gene core promoter), -hp53 (a 140 bp (−128–+12) gene core promoter) and -hPPM1D (a 849 bp gene core promoter) plasmids (see Materials and methods). According to (Wang and El-Deiry, 2006) p53 activates its own promoter, and (Lowe et al, 2010) nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activates the PPM1D promoter. In co-transfection experiments pcDNA3-p53 and pcDNA3-NF-κB/p65 triggered a >15-fold increase in pGL3-hp53 and pGL3-hPPM1D activity, respectively (Figure 1g). Co-transfection of pcDNA3-BRCA1-IRIS did not activate transcription from pGL3-hp53 or pGL3-hPPM1D, even though it triggered a >20-fold increase in pGL3-hCycD1 activity (Figure 1g). BRCA1-IRIS silencing (siGFP was used as negative control) in HME cells did not affect the activity of any of the promoters (Figure 1g).
BRCA1-IRIS enhances HuR expression and cytoplasmic localization
Whether BRCA1-IRIS triggers p53 and WIP1 expression by a post-transcriptional mechanism was studied next. Whole cell lysates, nuclear (soluble and chromatin-bound), polysomal (cytoplasmic and ribosomal-bound) proteins were isolated from BRCA1-IRIS-silenced MCF7 and SKBR3 (after 48 h), or HME and induced IRIS2 and IRIS3 cell lines (for 72 h). Compared with control, BRCA1-IRIS silencing in MCF7 and SKBR3 cells reduced the total HuR levels (Figure 2a), did not change nuclear HuR levels (Figure 2b, left), but significantly reduced cytoplasmic (Figure 2b, right) and polysomal HuR (Figure 2d, upper panels) levels. This was also evident in immunofluorescence analysis (Figure 2c). In contrast, compared with HME, induced IRIS2 and IRIS3 contained high total HuR levels (Figure 2e), similar nuclear HuR levels (Figure 2f, left), but higher cytoplasmic (Figure 2f, right) and polysomal HuR (Figure 2h, upper panels) levels. This was also evident in immunofluorescence analysis (Figure 2g), suggesting that BRCA1-IRIS stabilizes HuR in the cytoplasm rather than its cytoplasmic translocation. However, our attempts to measure differences in HuR polyubiquitination in BRCA1-IRIS-manipulated cells were unsuccessful.
BRCA1-IRIS induces the expression of p53 and WIP1 by a post-transcriptional mechanism. Expression of HuR, NF-κB/p65 in total lysates (a), nuclear (soluble and chromatin-bound) and cytoplasmic (b) extracts from BRCA1-IRIS-silenced MCF7 and SKBR3 cells. (c) HuR expression (green) in MCF7 and SKBR3-silenced cells, blue in DAPI-stained DNA. (d) HuR protein (upper) or PPM1D and p53 mRNAs (lower) in polysomal HuR immunoprecipitates of BRCA1-IRIS-silenced MCF7 and SKBR3 cells. Expression of HuR, NF-κB/p65 in total lysates (e), nuclear (soluble and chromatin-bound) and cytoplasmic (f) extracts from HME or induced IRIS2 and IRIS3. (g) HuR expression (green) in induced IRIS2 and IRIS3, blue is DAPI-stained DNA. (h) Immunoprecipitates of polysomal extracts done using HuR or immunoglobulinG (IgG) (control) antibodies. HuR protein (upper) or PPM1D and p53 mRNAs (lower) in polysomal HuR immunoprecipitates of HME or induced HME cells.
HuR transcription is NF-κB controlled (Kang et al., 2008). Compared with control, BRCA1-IRIS silencing reduced total NF-κB/p65 in MCF7 and SKBR3 cells (Figure 2a), whereas BRCA1-IRIS overexpression did not change total NF-κB/p65 (Figure 2e). BRCA1-IRIS silencing did not affect the level of nuclear NF-κB/p65 in MCF7 cells but decreased it in SKBR3 cells (Figure 2b). It increased the cytoplasmic NF-κB/p65 levels in MCF7 cells and decreased it slightly in SKBR3 cells (Figure 2b). On the other hand, BRCA1-IRIS overexpression increased nuclear NF-κB/p65 level, while reduced it in the cytoplasm, (Figure 2f) suggesting that in BRCA1-IRIS-silenced MCF7 cells, retention of NF-κB/p65 in the cytoplasm, signal the cells to downregulate the NF-κB/p65 expression, whereas in SKBR3 cells, NF-κB/p65 nuclear translocation is decreased. On the other hand, BRCA1-IRIS overexpression in HME cells triggers HuR expression by inducing NF-κB/p65 nuclear translocation. Whatever the mechanism, we propose that BRCA1-IRIS enhances HuR expression in a NF-κB/p65-dependent manner.
BRCA1-IRIS enhances HuR binding to p53 and PPM1D mRNAs
By using RT–PCR analysis on HuR polysomal immunoprecipitation we observed that compared with immunoglobulin G (negative control) immunoprecipitations that contained no detectable RNA in both cases (Figures 2d and h, lower panels), the polysomal HuR, immunoprecipitated from BRCA1-IRIS-silenced MCF-7 and SKBR3 cells contained lower levels of p53 and PPM1D mRNAs (Figure 2d). HuR immunoprecipitated from induced IRIS3 contained higher levels of p53 and PPM1D mRNAs (Figure 2h). As we detected no differences in nuclear (pre) p53 and PPM1D RNAs levels in BRCA1-IRIS-silenced MCF7 and SKBR3 or induced IRIS3 cells (Figures 2d and h), we propose that BRCA1-IRIS triggers p53 and WIP1 expression by enhancing their mRNA stability and/or translation in the cytoplasm by HuR.
BRCA1-IRIS silencing triggers p38MAPK and p53 activation by UVC
HME cells, with BRCA1-IRIS or p53 silenced (for 48 h), or treated with 10 μM of p38MAPK inhibitor, SB203580 (for 24 h) were exposed to a sub-lethal dose (20 mJ/cm2) of UVC or left untreated. In untreated cells, UVC induced BRCA1-IRIS, p53, p-p53 and WIP1 but not p38MAPK expression (Figure 3a). Interestingly, UVC increased p-p38MAPK level in HME but decreased it in MCF7 cells (compare Figures 3a–e). We reasoned that the different manipulation of cells in every experiment differentially affects the p53 and p38MAPK phosphorylation. Indeed, untreated MCF7 cells, grown for 24 h in no-serum or in the presence of lipofectamine or the media was removed for 1 min and then added back and left to grow for 24 h, showed differences in p-p53 and p-p38MAPK (Supplementary Figure 1b).
BRCA1-IRIS-induced WIP1 prevents p38MAPK/p53 activation by short-wavelength UV light (UVC) irradiation. Expression of indicated proteins in control (a), p53-silenced (b), p38-inactivated (c), BRCA1-IRIS-silenced cells (d) HME or MCF7 cells (e) exposed or not to 20 mJ/cm2 UVC. (f) Expression of indicated proteins in IRIS1 and induced IRIS3 exposed to increasing doses of UVC. (g) Percentage of phosphorylated p53 or p38 compared with total p53 or p38 in IRIS1 or induced IRIS3 exposed to increasing doses of UV. (h) Expression of the indicated proteins in HME and induced IRIS3 treated with 20 mJ/cm2 of UVC, 10 μM CCT007093 or both. *P<0.05 and **P<0.001.
In p53-silenced cells, UVC induced BRCA1-IRIS, WIP1 and p–p38MAPK expression, but had no effect on the total p38MAPK level (Figure 3b), in keeping with the data in Figure 1f, showing induction of WIP1 by a BRCA1-IRIS-dependent/p53-independent mechanism. In p38MAPK-inactivated cells, UVC induced BRCA1-IRIS, p53 and WIP1 but not p–p53 expression (Figure 3c), in keeping with p53 as a p38MAPK target in UVC-exposed cells (see above). Finally, in BRCA1-IRIS-silenced cells, UVC exposure slightly increased p53 and p-p53 levels yet no increase in WIP1 level was observed (Figure 3d), suggesting that BRCA1-IRIS is critical for UVC-induced WIP1 expression and/or stability. Moreover, p-p38MAPK level was much higher in these cells compared with vehicle-treated cells even before UVC treatment (compare unexposed lanes in Figures 3a and d) increased even further following UVC exposure (Figure 3d), perhaps due to geno-/cell-toxic stresses induced by BRCA1-IRIS silencing. This is further explained in the discussion.
BRCA1-IRIS overexpression abrogates UVC-induced p38MAPK/p53 activation in a WIP1-dependent manner
IRIS1 and IRIS3 induced for 72 h were exposed to 0, 10, 20 or 40 mJ/cm2 of UVC irradiation. After 24 h, compared with IRIS1, induced IRIS3 cells treated with 0 mJ/cm2 UVC had higher levels of BRCA1-IRIS, p53, WIP1, p-p38MAPK, yet similar levels of p38MAPK and p-p53 (Figure 3f). This is further explained in the discussion. In 10 and 20 mJ/cm2 treated cells, BRCA1-IRIS, p53 and WIP1 levels remained higher in induced IRIS3 and no change in p38MAPK level (Figure 3f). While in IRIS1 cells p-p53 and p-p38MAPK levels rose significantly, in IRIS3 p-p53 and p-p38MAPK levels declined (Figure 3f). Finally, in 40 mJ/cm2 UVC-treated cells, BRCA1-IRIS, p53 and WIP1 levels were still detectable in induced IRIS3 but not in IRIS1 cells (Figure 3f). In contrast, IRIS1 and not induced IRIS3 cells contained detectable levels of p-p53 and p-p38MAPK (Figure 3f). The intensity of the p53, p-p53, p38MAPK and p-p38MAPK bands in Figure 3f were measured using ImageJ program (NIH) to estimate the level of p53 and p38MAPK activation in BRCA1-IRIS overexpressing cells. About 50% of p53 in IRIS1 cells was phosphorylated before UVC exposure that rose to 80–100% after the exposure (Figure 3g). In contrast, only 20% of p53 was phosphorylated in induced IRIS3 cells before UVC dropping to <10% after UVC (Figure 3g). p38MAPK phosphorylation was undetectable in IRIS1 cells before UVC exposure but rose to ∼100% after UVC (Figure 3g). In induced IRIS3 cells, the 100% of p38MAPK phosphorylated before UVC exposure dropped to <20% after UVC exposure (Figure 3g).
To expand these data to other types of geno-/cell-toxic stresses, IRIS1 and induced IRIS2 (for 72 h) were treated with 0, 5, 10 or 50 μM of etoposide. After 24 h, IRIS1 cells showed low level of BRCA1-IRIS, p53 and WIP1 even after treatment with etoposide (Supplementary Figure 2). Induced IRIS2 on the other hand maintained high level of BRCA1-IRIS, p53 and WIP1 even in the presence of 50 μM of etoposide and had similar level of total p38MAPK (Supplementary Figure 2). Etoposide treatment triggered p53 phosphorylation in IRIS1 but not in the induced IRIS2 cells (Supplementary Figure 2). IRIS1 had low p-p38MAPK level before etoposide, increased significantly after etoposide treatment, whereas induced IRIS2 cells had high levels of p-p38MAKP before but sharply dropped after etoposide treatment. Taken together, these results suggest that BRCA1-IRIS overexpression prevents activation of p53 and/or p38MAPK by UVC and chemotherapeutic drugs.
WIP1 suppression restores p38MAPK/p53 activation in BRCA1-IRIS overexpressing cells
CCT007093 is a novel WIP1 specific inhibitor. The HME and induced IRIS3 (72 h) cells were untreated or exposed to 20 mJ/cm2 UVC, 10 μM of CCT007093 or both. BRCA1-IRIS, p53 and WIP1 level was low in HME and high in IRIS3 cells, whereas p38MAPK was high in both the cell lines. These levels were not changed in both cell lines following any of the treatments (Figure 3h). p-p53 level was low in untreated HME and IRIS3 cells (Figure 3h, lanes 1 and 5), rose after UVC treatment, decreased after CCT007093 treatment and increased in UVC + CCT007093-treated HME cells (Figure 3h). Importantly, in induced IRIS3 cells, p-p53 level decreased after UVC treatment, increased after CCT007093 treatment and remained high after the combined treatment (Figure 3h). Quantification of the ratios between p-p53 and p53 showed that in the presence of CCT007093, UVC induced phosphorylation of ∼75% of p53 in IRIS3 cells (Figure 3g). p-p38MAPK was not detected in untreated HME cells (Figure 3h, lanes 1 and 5), and was increased by UVC, CCT007093 and the combined treatment (Figure 3h). Importantly, in induced IRIS3, the p-p38MAPK level was high before UVC, dropped after UVC and remained high in cells treated with CCT007093 alone or in combination with UVC (Figure 3h). Quantification of the p-p38MAPK/p38MAPK ratio showed that in IRIS3 ∼55% of p38MAPK was phosphorylated by UVC in the presence of CCT007093 (Figure 3g). These data are in keeping with that BRCA1-IRIS abrogation of p53 and p38MAPK activation after UVC treatment is WIP1-dependent.
Inactivating p53 or p38MAPK protects against cell death induced by BRCA1-IRIS silencing, UVC, etoposide or H2O2 treatment
MCF7 and SKBR3 cell lines transfected with luciferase or BRCA1-IRIS siRNAs in the presence or absence of p38MAPK inhibitor (SB203580) or p53 siRNA were exposed to 40 mJ/cm2 UVC, 10 μM etoposide or 15 μM H2O2. Using LIVE/DEAD assay (Invitrogen, Carlsbad, CA, USA), we observed a significant increase in the number of dead cells 24 h after BRCA1-IRIS silencing in both the cell lines (Figure 4a and Supplementary Table 1). Inactivating p38MAPK or depleting p53 significantly reduced these effects (Figure 4a and Supplementary Table 1). This was confirmed by phase-contrast cell counting (Supplementary Figure 3a) and trypan blue exclusion using ViCell (Beckman Coulter, Fullerton, CA, USA, Supplementary Figure 3b). UVC, etoposide or H2O2 treatment increased the level of cell death observed in all conditions, but the trend remained the same (Figures 4b, c and d, Supplementary Figures 3a and b). Moreover, using the Apo-ONE Homogeneous Caspase-3/7 assay (Promega, Madison WI, USA) we observed an increase in active caspase 3/7 levels in BRCA1-IRIS-silenced cells alone or in combination with UVC, etoposide or H2O2 treatment in both cell lines, and that p38MAPK inhibition or p53 silencing significantly reduces that level (Supplementary Figure 3c). Consistently, BRCA1-IRIS silencing alone or in combination with etoposide or H2O2 increased the level of cleaved poly-(ADP ribose) polymerase, the caspase-3 target during cell death (Nicholson and Thornberry, 1997), in both cell lines (Figure 4e).
BRCA1-IRIS silencing enhances the killing of HME cells by geno-/cell-toxic stresses. Percentage of dead cells in control, BRCA1-IRIS-silenced, BRCA1-IRIS-silenced/p38-inactivated or BRCA1-IRIS silenced/p53 silenced MCF7 cells treated with (a) nothing, (b) 40 mJ/cm2, (c) 10 μM etoposide or (d) 15 μM H2O2as detected by LIVE/DEAD analysis. (e) Levels of cleaved poly-(ADP ribose) polymerase in control and BRCA1-IRIS-silenced MCF7 and SKBR3 cells after none, 10 μM etoposide or 15 μM H2O2 treatment. *P<0.05 and **P<0.001.
BRCA1-IRIS protects cells from UVC-, etoposide- and H2O2-induced apoptosis by suppressing p53 and p38MAPK activities
HME and induced IRIS3 (72 h) cells were exposed to 40 mJ/cm2 UVC, 10 μM of etoposide or 15 μM of H2O2 in the presence or absence of 10 μM of SB203580, p53 siRNA or 10 μM of CCT007093. By using a Fluorescein FragEL DNA fragmentation detection kit to detect TUNEL+ cells, we found that under no treatment conditions, CCT007093 increased slightly yet significantly the number of TUNEL+ cells in HME, but not in induced IRIS3 cells (Figure 5). Treatment with UVC, etoposide or H2O2 markedly increased the numbers of TUNEL+ cells in HME cells, while only modestly increasing the numbers in IRIS3 cells (Figure 5 and Supplementary Figures 4a–f). p38MAPK inhibition or p53 silencing significantly reduced the number of TUNEL+ cells under all treatment conditions in HME, but it stayed the same in induced IRIS3 cells (Figure 5 and Supplementary Figures 4g–r). Compared with vehicle control, CCT007093 treatment significantly increased the number of TUNEL+ cells in induced IRIS3 and not in HME cells (compare black with white bars in Figure 5 and see Supplementary Figures 4s-x). This suggests that BRCA1-IRIS overexpression protects against geno-/cell-toxic stress-induced apoptosis by preventing p53 and p38MAPK activation in a WIP1-dependent manner.
BRCA1-IRIS overexpression induces proliferation of UVC-damaged cells
Equal numbers of HME, IRIS2 or IRIS3 cells were grown in the presence of 2 μg/ml doxycycline and treated with 10 μM SB203580 or transfected with p53 siRNA or both before they were treated with 20 mJ/cm2 UVC. At 24 h, MTT assay showed that BRCA1-IRIS overexpression alone increased cell viability after UVC treatment (compare bars 2 and 3 with 1 in Supplementary Figure 5). Blocking UVC-induced apoptosis by silencing p53 or inactivating p38MAPK or both in HME cells increased the viability compared with UVC-untreated cells (compare bars 4, 7 and 10 with 1 in Supplementary Figure 5). p53 silencing, p38MAPK inactivation or the combination increased even further the viability of IRIS2 and IRIS3 cells exposed to UVC (compare bars 5 and 6 to 4, bars 8 and 9 with 7 and 11 and 12 to 10 in Supplementary Figure 5). This suggests that BRCA1-IRIS overexpression promotes proliferation of UVC-damaged cells by suppressing p53 and/or p38MAPK expression or activity in a WIP1-dependent manner.
BRCA1-IRIS overexpression transforms HME cells with WIP1 or oncogenic Ras
WIP1 cooperates with oncogenic Ras (RasV12) in transforming mammalian cells (Harrison et al., 2004). Whether BRCA1-IRIS also cooperates with WIP1 or RasV12 in transforming HME cells was studied next. Empty (control), RasV12 or WIP1-expressing vectors were transfected into IRIS1, IRIS2 or IRIS3 cell lines and cells were induced and counted 72 h later, or were plated on soft agar plates, maintained in doxycycline and the colony size and number formed in each culture was measured 2–3 weeks later.
BRCA1-IRIS overexpression triggered two- to three-fold increase in cell number compared with control (Figure 6a). RasV12 and WIP1 overexpression (done in parental HME cells) each induced ∼three-fold increase, while the combination led to >10-fold increase (Figure 6a). RasV12 or WIP1 overexpression in IRIS1 induced a modest two- to three-fold increase (Figure 6a). Whereas RasV12 or WIP1 overexpression in induced IRIS2 and IRIS3 cells led to eight- to ten-fold increase in cell number (Figure 6a), suggesting that BRCA1-IRIS cooperates with RasV12 or WIP1 in inducing HME cell proliferation.
BRCA1-IRIS transforms HME cells in combination with WIP1 or oncogenic Ras (RasV12). (a) The number of cells in IRIS1 and induced IRIS2 or IRIS3 transfected with nothing, pcDNA3-RasV12 or pcDNA3-WIP1. Data presented as mean±s.d. from three separate experiments done in triplicates. (b) Number of colonies of IRIS1 and induced IRIS3 transfected with pcDNA3-RasV12 or pcDNA3-WIP1 growing in soft agar coated wells. Data presented as mean±s.d. from three separate experiments done in triplicates. +P⩽0.5 compared with IRIS1. In both panels, *P<0.05 and **P<0.001.
In the soft agar experiments, while a low number of small-sized colonies was observed in the IRIS1 transfected with RasV12 or WIP1 alone (Figure 6b and Supplementary Figure 6), transfection of either plasmid in induced IRIS2 or IRIS3 cells led to a greater number of larger-sized colonies (Figure 6b and Supplementary Figure 6), suggesting that BRCA1-IRIS transforms HME cells with RasV12 or WIP1, in vitro.
Discussion
We report that BRCA1-IRIS overexpression inactivates p38MAPK and p53 by inducing the expression of their WIP1 phosphatase, that BRCA1-IRIS triggering p53 and WIP1 expression is post-transcriptional, BRCA1-IRIS induces PPM1D and p53 mRNA stabilization and translation by promoting the expression and cytoplasmic availability of the mRNA-binding protein, HuR. This is in keeping with its ability to induce WIP1 expression in mutant as well as in wild-type p53 cells. Although HuR binding and stabilization of p53 mRNA has been reported earlier (Al-Mohanna et al., 2007; Tong and Pelling, 2009), we are the first to show post-transcriptional control of WIP1 expression by HuR.
HuR levels are high in gastric, breast, pancreatic, colon and oral tumors (Hasegawa et al., 2009; Licata et al., 2009; Lim et al., 2009; Maréchal and Van Laethem, 2009), and in tumor cell lines no genetic or epigenetic alterations in the HuR gene have been found. Kang et al. (2008) recently showed that HuR overexpression depends on PI3‘K/AKT signaling and NF-κB activity, and that AKT activation increased p65/RelA binding to a putative NF-κB-binding site in the HuR promoter. We recently observed that BRCA1-IRIS overexpression induces AKT1 and AKT2 expression and stabilization in HME as well as ovarian surface epithelial cells (ElShamy, submitted). Together with the data presented here, it is tempting to speculate that BRCA1-IRIS triggers HuR expression in a AKT/NF-κB-dependent manner and promotes aggressive breast and perhaps other cancers.
Activated p38MAPK induces checkpoints that arrest cells in G1/S or G2/M to allow time for repair pathways (Mikhailov et al., 2004; Reinhardt et al., 2007), in part by phosphorylating p53 (Huang et al., 1999; She et al., 2001) leading to the transcription of target genes such as Gadd45α, p21 and 14-3-3 (El-Deiry et al., 1993; Hermeking et al., 1997; Jin et al., 2003). Unlike cancer cells, BRCA1-IRIS silencing or overexpression activated the p38MAPK in HME cells. It is possible that in BRCA1-IRIS-silenced cells the induction of replication arrest (ElShamy and Livingston, 2004) activates p38MAPK, as was shown recently (Im and Lee, 2008; Rodríguez-Bravo et al., 2007). However, in undamaged BRCA1-IRIS overexpressing cells, perhaps p38MAPK is activated to induce cell survival and/or motility (Suarez-Cuervo et al., 2004; Demuth et al., 2007; Hsieh et al., 2007; Junttila et al., 2007). Alternatively, as p-p38MAPK phosphorylates HuR in the nucleus and enhances its cytoplasmic translocation (Gorospe, 2003; Tran et al., 2003; Lafarga et al., 2009), it is possible that in undamaged cells, this is how BRCA1-IRIS induces HuR cytoplasmic translocation (this study). If true in vivo, then this could be a reason to develop an anti-IRIS-based therapy.
Materials and methods
Cell culture and UVC, etoposide and H2O2 treatments
BT474, MCF7 and SKBR3 cells were maintained in Roswell Park Memorial Institute medium (Invitrogen) supplemented with 10% fetal bovine serum and antibiotics. SOAS2 and HEK293T cells were maintained Dulbecco's Modified Eagle medium (Invitrogen) with 10% serum. HME maintenance was as described in ElShamy and Livingston, 2004. Cancer or HME cells were at 80% confluence in 10 cm2 dishes and were treated with UVC (different doses), SB203580 (10 μM, Sigma, St Louis, MO, USA), etoposide (10 μM, Sigma) and H2O2 (15 μM, Sigma) or CCT007093 (10 μM, Santa Cruz, Santa Cruz, CA, USA), and these drugs were added directly to the cells.
Antibodies
BRCA1-IRIS monoclonal antibody was produced in our laboratory. Sampler kits for p53 (#9919) and p38MAPK (#9913) were from Cell Signaling (Danvers, MA, USA). p-p53 was detected individually or by using mixtures of several phospho-specific p53 (S15), (S37) and (S46) supplied in this kit. p-p38MAPK antibody was produced against p-T180/p-Y182 supplied in the kit. Mouse anti-HuR (#39-0600) and rabbit anti-cleaved poly-(ADP ribose) polymerase (# 44698G) antibodies were from Invitrogen. Lamin B1 (Oncogene, Boston, MA, USA, # NA12-100UG), NF-κB/p65 (Santa Cruz, C-20), WIP1 (Abgent, San Diego, CA, USA, # AP84370) and Actin (Calbiochem, Gibbstown, NJ, USA, chem, # CP01) were used.
Reporters gene construction
The p53 promoter report construct design was according to (Li et al., 2007). The resultant plasmids were named as pGL3-hp53. For details see Materials and methods Supplementary Information. The PPM1D promoter report construct was designed according to standard protocol. The resultant plasmid was named as pGL3-hPPM1D. For details see Materials and methods Supplementary Information. The pGL3-hCycD1 construct was described earlier (Nakuci et al., 2006). For construction of expression plasmids see Materials and methods Supplementary Information.
Transient transfection
Cells were transiently transfected using Lipofectamine PLUS reagent (Invitrogen). For detail protocol see Materials and methods in Supplementary Information.
Stable transfection
The plasmids pMKO.1-puro (control) or pMKO.1-shp53-puro were obtained from Addgene (Cambridge, MA, USA). Retrovirus production and infection into MCF7 cells was done using standard protocols. For more details see Materials and methods in Supplementary Information.
Cell fractionation
To prepare cytoplasmic, nuclear and polysomal fractions the protocol of (Tenenbaum et al., 2002) was used. For detail protocol see Materials and methods in Supplementary Information.
Immunofluorescence
The protocol described in ElShamy and Livingston was used. For detail protocol see Materials and methods in Supplementary Information.
RT–PCR assays
Total RNA was isolated after treatments using TRIzol reagent (Invitrogen) and treated with a DNA-free kit (Ambion, Austin, TX, USA) to eliminate genomic DNA contamination. SuperScript III One-step RT-PCR with Platinum Taq (Invitrogen). For primers see Materials and methods in Supplementary Information.
Immunoprecipitation of mRNP-protein complex and RT–PCR
The protocol used by (Tenenbaum et al., 2002) was used. For detail protocol see Materials and methods in Supplementary Information.
RNA interference experiment
A dAdA-N19 double-stranded siRNA BRCA1-IRIS oligomer was synthesized and corresponded to a specific segment of the intron-11 sequence (available on request). A double-stranded 19-nucleotide luciferase- or green fluorescent protein-specific siRNAs was used as a negative control (Dharmacon, Lafayette, CO, USA). Commercial p53 and WIP1 siRNA (Dharmacon) were used. The transfection of siRNA in breast cancer as well as HME cells was performed using Oligofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Cells were harvested for 48or 72 h after transfection.
Cell death by fluorescence-activated cell sorting analysis
The LIVE/DEAD Fixable Dead Cell Stain Kit was used according to the supplier (Invitrogen) protocol.
TUNEL detection protocol
The Flurescein FragEL DNA Fragmentation Detection Kit was used according to the supplier (Calbiochem) protocol.
Active Caspase 3/7 detection protocol
The Apo-ONE Homogeneous Caspase-3/7 Kit was used according to the supplier (Promega) protocol.
MTS assay
CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay Kit (Promega) was used following the supplier instructions. As BRCA1-IRIS is a known inducer of replication and proliferation (ElShamy and Livingston, 2004; Nakuci et al., 2006; Hao and ElShamy, 2007), data are presented as (X treatment with UVC)/(X treatment without UVC)/(untreated HME with UVC)/(untreated HME without UVC), where X=given treatment.
Soft agar colony formation assay
Cells cultivated for 6 days with or without doxycycline (2 μg/ml) were plated in 0.3% agar (with or without doxycycline) on the plates, containing 0.5% agar (with or without doxycycline). The cells were cultivated on the plates for 2–3 weeks. Crystal violet stained colonies were counted under the microscope. Samples were assayed in triplicates.
Statistical analysis
Comparisons of treatment outcomes were tested for statistical differences using the Student's t-test for paired data. Statistical significance was assumed at a P-value of <0.05.
References
Al-Mohanna M, Al-Khalaf H, Al-Yousef N, Aboussekhra A . (2007). The p16INK4a tumor suppressor controls p21WAF1 induction in response to ultraviolet light. Nucleic Acids Res 35: 223–233.
Bulavin D, Phillips C, Nannenga B, Timofeev O, Donehower L, Anderson C et al. (2004). Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat Genet 36: 343–350.
Demuth T, Reavie L, Rennert J, Nakada M, Nakada S, Hoelzinger D et al. (2007). MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3 and p38 drive glioma invasion and progression and predict patient survival. Mol Cancer Ther 6: 1212–1222.
Dumaz N, Meek D . (1999). Serine15 phosphorylation stimulates p53 transactivation but does not directly influence interaction with HDM2. EMBO J 18: 7002–7010.
ElShamy WM, Livingston DM . (2004). Identification of BRCA1-IRIS, a BRCA1 locus product. Nat Cell Biol 6: 954–967.
El-Deiry W, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM et al. (1993). WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817.
Fiscella M, Zhang H, Fan S, Sakaguchi K, Shen S, Mercer W et al. (1997). Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci USA 94: 6048–6053.
Fornace Jr A . (1999). Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J 18: 6845–6854.
Gorospe M . (2003). HuR in the mammalian genotoxic response: post-transcriptional multitasking. Cell Cycle 2: 412–414.
Hammond E, Giaccia A . (2005). The role of p53 in hypoxia-induced apoptosis. Biochem Biophys Res Commun 331: 718–725.
Hao L, ElShamy WM . (2007). BRCA1-IRIS activates cyclin D1 expression in breast cancer cells by downregulating the JNK phosphatase DUSP3/VHR. Int J Cancer 121: 39–46.
Harrison M, Li J, Degenhardt Y, Hoey T, Powers S . (2004). Wip1-deficient mice are resistant to common cancer genes. Trends Mol Med 10: 359–361.
Hasegawa H, Kakuguchi W, Kuroshima T, Kitamura T, Tanaka S, Kitagawa Y et al. (2009). HuR is exported to the cytoplasm in oral cancer cells in a different manner from that of normal cells. Br J Cancer 100: 1943–1948.
Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S et al. (1997). 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell 1: 3.
Hickson JA, Fong B, Watson PH, Watson AJ . (2007). PP2Cdelta (Ppm1d, WIP1), an endogenous inhibitor of p38 MAPK, is regulated along with Trp53 and Cdkn2a following p38 MAPK inhibition during mouse preimplantation development. Mol Reprod Dev 74: 821–834.
Hirasawa A, Saito-Ohara F, Inoue J, Aoki D, Susumu N, Yokoyama T et al. (2003). Association of 17q21-q24 gain in ovarian clear cell adenocarcinomas with poor prognosis and identification of PPM1D and APPBP2 as likely amplification targets. Clin Cancer Res 9: 1995–2004.
Hsieh Y, Wu T, Huang C, Hsieh Y, Hwang J, Liu J . (2007). p38 mitogen-activated protein kinase pathway is involved in protein kinase Calpha-regulated invasion in human hepatocellular carcinoma cells. Cancer Res 67: 4320–4327.
Huang C, Ma WY, Maxiner A, Sun Y, Dong Z . (1999). p38 kinase mediates UV-induced phosphorylation of p53 protein at serine 389. J Biol Chem 274: 12229.
Im JS, Lee JK . (2008). ATR-dependent activation of p38 MAP kinase is responsible for apoptotic cell death in cells depleted of Cdc7. J Biol Chem 283: 25171–25177.
Jin S, Mazzacurati L, Zhu X, Tong T, Song Y, Shujuan S et al. (2003). Gadd45a contributes to p53 stabilization in response to DNA damage. Oncogene 22: 8536.
Junttila M, Ala-Aho R, Jokilehto T, Peltonen J, Kallajoki M, Grenman R et al. (2007). p38alpha and p38delta mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene 26: 5267–5279.
Kang M, Ryu B, Lee M, Han J, Lee J, Ha T et al. (2008). NF-kappaB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology 135: 2030–2042, 2042.e1-3.
Lafarga V, Cuadrado A, Lopez de Silanes I, Bengoechea R, Fernandez-Capetillo O, Nebreda A . (2009). p38 Mitogen-activated protein kinase- and HuR-dependent stabilization of p21(Cip1) mRNA mediates the G/S checkpoint. Mol Cell Biol 29: 4341–4351.
Latonen L, Laiho M . (2005). Cellular UV damage responses—functions of tumor suppressor p53. Biochim Biophys Acta 1755: 71–89.
Li I, Ke S, Ruiwen C, Yan H, Dan W, Shuhan S . (2007). p53 promoter-based reporter gene in vitro assays for quick assessment of agents with genotoxic potential. Acta Biochim Biophys Sin 39: 181–186.
Licata L, Hostetter C, Crismale J, Sheth A, Keen J . (2009). The RNA-binding protein HuR regulates GATA3 mRNA stability in human breast cancer cell lines. Breast Cancer Res Treat 122: 55–63.
Lim S, Lee S, Joo S, Song J, Choi S . (2009). Cytoplasmic Expression of HuR is Related to Cyclooxygenase-2 Expression in Colon Cancer. Cancer Res Treat 41: 87–92.
Lowe SW . (1999). Activation of p53 by oncogenes. Endocr Relat Cancer 6: 45–48.
Lowe J, Cha H, Yang Q, Fornace Jr A . (2010). Nuclear Factor-κB (NF-κB) Is a Novel Positive Transcriptional Regulator of the Oncogenic Wip1 Phosphatase. J Bio Chem 285: 5249–5257.
Maréchal R, Van Laethem J . (2009). HuR modulates gemcitabine efficacy: new perspectives in pancreatic cancer treatment. Expert Rev Anticancer Ther 9: 1439–1441.
Mazan-Mamczarz K, Galba'n S, Lo'pez de Silanes I, Martindale J, Atasoy A, Keene J et al. (2003). RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc Natl Acad Sci 100: 8354–8359.
Mikhailov A, Shinohara M, Rieder C . (2004). Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK checkpoint pathway. J Cell Biol 166: 517.
Moll U, Schramm L . (1998). p53—an acrobat in tumorigenesis. Crit Rev Oral Biol Med 9: 23–37.
Nakuci E, Mahner S, Direnzo J, ElShamy WM . (2006). BRCA1-IRIS regulates cyclin D1 expression in breast cancer cells. Exp Cell Res 312: 3120–3131.
Nicholson DW, Thornberry NA . (1997). Caspases: killer proteases. TIBS 22: 299–306.
Reinhardt H, Aslanian A, Lees J, Yaffe M . (2007). p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell 11: 175.
Rodríguez-Bravo V, Guaita-Esteruelas S, Salvador N, Bachs O, Agell N . (2007). Different S/M checkpoint responses of tumor and non tumor cell lines to DNA replication inhibition. Cancer Res 67: 11648–11656.
Saito-Ohara F, Imoto I, Inoue J, Hosoi H, Nakagawara A, Sugimoto T et al. (2003). PPM1D is a potential target for 17q gain in neuroblastoma. Cancer Res 63: 1876–1883.
Sakuma T, Nakagawa T, Ido K, Takeuchi H, Sato K, Kubota T . (2008). Expression of vascular endothelial growth factor-A and mRNA stability factor HuR in human meningiomas. J Neurooncol 88: 143–155.
She Q, Bode AM, Ma WY, Chen NY, Dong Z . (2001). Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res 61: 1604.
Shieh S, Ikeda M, Taya Y, Prives C . (1997). DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91: 325–334.
Sturgill T . (2008). MAP kinase: it's been longer than fifteen minutes. Biochem Biophys Res Commun 371: 1–4.
Suarez-Cuervo C, Merrell MA, Watson L, Harris KW, Rosenthal EL, Väänänen HK et al. (2004). Breast cancer cells with inhibition of p38alpha have decreased MMP-9 activity and exhibit decreased bone metastasis in mice. Clin Exp Metastasis 21: 525.
Sykes SM, Stanek TJ, Frank A, Murphy ME, McMahon SB . (2009). Acetylation of the DNA binding domain regulates transcription-independent apoptosis by p53. J Biol Chem 284: 20197–20205.
Takekawa M, Adachi M, Nakahata A, Nakayama I, Itoh F, Tsukuda H et al. (2000). p53-inducible Wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J 19: 6517–6526.
Tenenbaum SA, Lager P, Carson C, Keene J . (2002). Ribonomics: identifying mRNA subsets in mRNP complexes using antibodies to RNA-binding proteins and genomic arrays. Methods 26: 191–198.
Tong X, Pelling J . (2009). Enhancement of p53 Expression in Keratinocytes by the Bioflavonoid Apigenin is Associated With RNA-Binding Protein HuR. Mol Carc 48: 118–129.
Tran H, Maurer F, Nagamine Y . (2003). Stabilization of urokinase and urokinase receptor mRNAs by HuR is linked to its cytoplasmic accumulation induced by activated mitogen-activated protein kinase-activated protein kinase 2. Mol Cell Biol 23: 7177–7188.
Wang S, El-Deiry WS . (2006). p73 or p53 directly regulates human p53 transcription to maintain cell cycle checkpoints. Cancer Res 66: 6982–6989.
Yamaguchi H, Minopoli G, Demidov O, Chatterjee D, Anderson C, Durell S et al. (2005). Substrate specificity of the human protein phosphatase 2C delta, Wip1. Biochemistry 44: 5285–5294.
Yao KM, Samson ML, Reeves R, White K . (1993). Gene elav of Drosophila melanogaster: a prototype for neuronal-specific RNA binding protein gene family that is conserved in flies and humans. J Neurobiol 24: 723–739.
Acknowledgements
This research was supported in part by a start-up support from the University of Hawaii, and in part by a Grant from the Hawaii Community Foundation to WMeS. Wael M ElShamy is a Dr Lawrence and Mrs Bo Hing Chen Tsue American Cancer Society Scholar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
We declare there are not any competing financial interests in relation to the study described. However, Wael M ElShamy has submitted patent application.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Chock, K., Allison, J. & ElShamy, W. BRCA1-IRIS overexpression abrogates UV-induced p38MAPK/p53 and promotes proliferation of damaged cells. Oncogene 29, 5274–5285 (2010). https://doi.org/10.1038/onc.2010.262
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.262
Keywords
This article is cited by
-
Molecular characterization and elucidation of the function of Hap38 MAPK in the response of Helicoverpa armigera (Hübner) to UV-A stress
Scientific Reports (2022)
-
Molecular characterization of two MAPK genes and their thermotolerant functions in a high temperature acclimated strain of Neoseiulus barkeri
BioControl (2022)
-
Premature polyadenylation of MAGI3 is associated with diminished N6-methyladenosine in its large internal exon
Scientific Reports (2018)
-
The role of BRCA1-IRIS in the development and progression of triple negative breast cancers in Egypt: possible link to disease early lesion
BMC Cancer (2017)
-
BRCA1-IRIS inactivation overcomes paclitaxel resistance in triple negative breast cancers
Breast Cancer Research (2015)