Abstract
The molecular basis for the resistance of tumor cells to cell-mediated cytotoxicity remains poorly understood and thus poses a major challenge for cancer immunotherapy. The present study was designed to determine whether the WNT1-inducible signaling pathway protein 2 (WISP2, also referred to as CCN5), a key regulator of tumor cell plasticity, interferes with tumor susceptibility to cytotoxic T-lymphocyte (CTL)-mediated lysis. We found that silencing WISP2 signaling in human breast adenocarcinoma MCF7 cells impairs CTL-mediated cell killing by a mechanism involving stem cell marker Kruppel-like factor-4 (KLF-4) induction and microRNA-7 (miR-7) downregulation. Inhibition of transforming growth factor beta (TGF-β) signaling using the A83-01 inhibitor in MCF7-shWISP2 cells resulted in a significant reversal of the epithelial-to-mesenchymal-transitioned (EMT) phenotype, the expression of KLF-4 and a partial recovery of target susceptibility to CTLs. More importantly, we showed that silencing KLF-4 was accompanied by a reduction in MCF7-shWISP2 resistance to CTLs. Using human breast cancer tissues, we demonstrated the coexpression of KLF-4 with EMT markers and TGF-β pathway signaling components. More importantly, we found that KLF-4 expression was accompanied by miR-7 inhibition, which is partly responsible for impairing CTL-mediated lysis. Thus, our data indicate that WISP2 has a role in regulating tumor cell susceptibility through EMT by inducing the TGF-β signaling pathway, KLF-4 expression and miR-7 inhibition. These studies indicate for the first time that WISP2 acts as an activator of CTL-induced killing and suggests that the loss of its function promotes evasion of immunosurveillance and the ensuing progression of the tumor.
Similar content being viewed by others
Introduction
Although the identification of tumor-associated antigens provided a new arsenal of approaches to enhance antigen-specific immune responses, the immunotherapeutical approaches that are currently used in the clinic have only had limited success. The ultimate goal of most of the reported immunotherapy strategies is to induce a strong cytotoxic T-lymphocyte (CTL) response. The prevailing view is that induced CTLs will eradicate tumor cells. However, this view has been seriously challenged by clinical observations showing that, even if a strong and sustained cytotoxic response is induced, complex issues, such as tumor evasion, persist and the selection of immune-resistant tumor cell variants remain.1 The coexistence of tumor cells and tumor-specific circulating CD8+ T cells in cancer patients remains an intriguing paradox in tumor immunology. Extensive studies have shown that tumor cells themselves have a crucial role in modulating the host immune response so that they can maintain their functional disorder and evade immune surveillance.2 In this regard, it has been suggested that tumor cell growth in vivo is not only influenced by CTL-tumor cell recognition but also by tumor susceptibility to cell-mediated death.3 Although the resistance of tumor cells to cell-mediated cytotoxicity remains a major impediment for cancer immunotherapy, its molecular basis is poorly understood. Resistance to CTL-mediated cytotoxicity has been attributed to several possible mechanisms,4 and growing evidence suggests a gain of plasticity is a new tumor adaptation to escape immunological strategies during tumor progression.5 In this regard, epithelial-to-mesenchymal transition (EMT), a de-differentiation process implicated in metastasis and the generation of cancer-initiating cells in solid tumors, has been reported to involve the activation of several important pathways that help tumors survive, grow and evolve into highly invasive and metastatic variants.6
WNT1-inducible signaling pathway protein 2 (WISP2)/CCN5 is constitutively expressed in less-aggressive human breast cancer cell lines, but minimally detected in moderately aggressive lines and undetectable in highly aggressive lines.7,8 It has been suggested that WISP2 has a role in the maintenance of a differentiated and non-invasive phenotype in human breast cancer cells,8 and reports indicate that a loss of WISP2 activity may promote breast cancer progression.9, 10, 11 However, WISP2 has both growth-promoting and growth-arresting competence, depending on the cell type and the cellular microenvironment.12 There is significant experimental evidence to show that WISP2 has an anti-invasive role in carcinogenesis through controlling adhesion and cell motility8,9 and interfering with EMT. In previous studies, we showed that EMT coordinately regulates target cell recognition and sensitivity to specific lysis by promoting autophagy,13,14 suggesting that EMT may represent a critical target for the development of novel immunotherapeutic approaches. Although the role of WISP2 in oncogenesis has been well characterized, its putative role in the regulation of the immune system remains unknown.
In consideration of these previous findings, we sought to determine whether WISP2 exerts its tumor growth control function by interfering with the anti-tumor cytotoxic response. Our data indicate that WISP2 may orchestrate a dynamic molecular cross-talk between killer cells and target cells that has implications for recognition of the tumor by immune cells and in shaping tumor target sensitivity to CTL-induced cell death.
Results
Silencing WISP2 activates TGF-β signaling and induces EMT, as respectively revealed by Single Sample Geneset Enrichment Analysis (ssGSEA) and EMT scoring
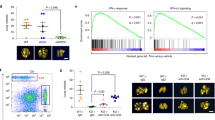
WISP2 has been reported as a transcriptional repressor of the transforming growth factor beta (TGF-β) signaling pathway.10 WISP2, associated with HDAC1, is recruited to the TGF-β-receptor-II promoter to restrict its transcription.10 Western blot analysis showed a gain in the expression of EMT markers SNAI1 (Snail), SNAI2 (Slug), Twist, Zeb1, Vimentin and N-cadherin for MCF7 with shWISP2 (Figure 1a). In addition, immunofluorescence analysis showed downregulation of the epithelial markers E-cadherin, β-catenin, ZO-1 and cytokeratin 18 in the MCF7-shWISP2 cell line compared with MCF7 parental cells and -shScrambled cells (Figure 1b). To evaluate the influence of WISP2 silencing on the degree of activation of TGF-β signaling, we performed Single Sample Geneset Enrichment Analysis15 using four gene sets related to TGF-β signaling (Figure 1c) from Msigdb v3.0.16 We found that TGF-β signaling was increased significantly in WISP2 knockdown cells as compared with the parental MCF7 or MCF7-shScrambled (Mann–Whitney U-test, P=0.004). We next used the EMT scoring method that we have recently developed13 to quantitatively estimate the effect of WISP2 silencing on the degree of ‘EMT-ness’ of MCF7 cells, with epithelial cells receiving an EMT score of −1.0, and mesenchymal cells a score of +1.0. To position parental MCF7, MCF7-shScrambled and MCF7-shWISP2 cells in such an EMT gradient, we computed their EMT scores alongside those of various breast cancer cell lines belonging to two collections, namely E-TABM-15717 and GSE15026 (Duke collection (http://www.ncbi.nlm.nih.gov/pubmed/19483725)). A large shift was observed in the EMT score for MCF7-shWISP2 cells (+0.34) as compared with those for the MCF7 parental (−0.97) and -shScrambled- (−1.0) lines (Figure 1d). This finding demonstrates that WISP2 knockdown MCF7 cells acquired a mesenchymal-like phenotype in contrast to the epithelial-like MCF7 parental or -shScrambled cells.
Silencing WISP2 activates TGF-β signaling and induces epithelial-mesenchymal transition (EMT) as revealed by western blot, immunofluorescence, Single Sample Geneset Enrichment Analysis (ssGSEA) and EMT scoring. (a) Western blot analysis of EMT markers. The expression of each EMT marker was determined using the indicated antibodies. (b) Immunofluorescence staining of F-actin and epithelial markers (E-cadherin, β-catenin, Zonula Occludens-1 (ZO-1) and cytokeratin 18). Nuclei were stained with 4',6-diamidino-2-phenylindole (blue), Bar=20 μm. (c) Bar plot of estimated TGF-β signaling activity in parental MCF7, MCF7-shScrambled and MCF7-shWISP2. Colors of the bars indicate the gene sets used and the y-axis indicates the enrichment score as computed by ssGSEA. (d) Bar plot of EMT score of breast cancer cell lines from data generated in this study, E-TABM-157 (labeled ‘Gray’) and GSE15026 (labeled ‘Duke’). The breast cancer cell lines were aligned from the most epithelial (EMT score=−1.0) to the most mesenchymal (EMT score=+1.0). Various breast cancer cell lines are named above or below the bar plot. Positions of data generated in this study, parental MCF7, MCF7-shScrambled and MCF7-shWISP2 are indicated in black (arrows). Breast cancer molecular subtypes were assigned to each line according to Neve et al.17 Maroon, red and blue colors represent basal-A, basal-B and luminal subtypes, respectively.
Downregulation of HLA-A2 and TAP1/2 expression, and altered CTL reactivity by silencing WISP2
To examine the functional consequences of WISP2-repressed TGF-β signaling in MCF7 cells, we tested the susceptibility of shRNA-treated MCF7 cell lines to lysis by the H33 CTL clone, which can lyse the parental MCF7 cell line in a HLA-A2-restricted manner.18 As shown in Figure 2A, we observed a significant decrease in MCF7-shWISP2 cell lysis as compared with MCF7 parental or MCF7-shScrambled cells. We also found a similar decrease in specific cell lysis when MDA-MB-231 cells, which do not express or secrete WISP2, were used as target cells (Supplementary Figure 1A).
Downregulation of HLA-A2, TAP1/2 expression and alteration of CTL reactivity by silencing WISP2. (A) Cytotoxicity was determined by a conventional 4 h 51Cr release assay at different effector:target (E:T) ratios on 1000 target cells per well. Bars indicate the mean, and the error bars indicate the s.d. (B) (a) Decrease in HLA-A2 molecule expression on the surfaces of the EMTed cells. (b) Western blot analysis of TAP1 and TAP2 expression using the indicated Abs. (c) TNF-β production by the T cell clone in response to tumor cell stimulation. Significant P-values are indicated by asterisks (*P=0.03; **P=0.003; ***P<0.001).
On the basis of these data, we next sought to determine whether the decrease in target killing interferes with CTL reactivity. For this purpose, we first tested the influence of WISP2 loss on HLA-A2 expression. Fluorescence-activated cell sorting analysis indicated a significant decrease in HLA-A2 expression in MCF7-shWISP2 cells (Figure 2B (a)) as compared with the MCF7-shScrambled and parental cells. Furthermore, western blotting analysis showed that both TAP1 and TAP2 expression were downregulated in MCF7-shWISP2 cells as compared with the control cells (Figure 2B (b)). More importantly, co-culture of the H33 CTL clone with MCF7-shWISP2 resulted in a significant inhibition of TNF-β secretion by this clone (Figure 2B (c)). Collectively, these data indicate that WISP2 silencing interferes with the cross-talk between CTLs and their target and induces an alteration in CTL reactivity.
Selective induction of KLF4 in MCF7-shWISP2 and alleviated resistance to CTLs following KLF4 silencing
Previous studies have indicated that the expression of the stemness transcription factor Nanog regulates cell susceptibility to CTL-mediated cell killing.19 We therefore examined the effect of WISP2 silencing on the acquisition of stem cell-like properties, such as the expression of stem cell transcription factors SOX2, OCT4, Nanog, CD133 and KLF4. Results from quantitative real-time (qRT)–PCR showed a lack of increase in the expression of these transcription factors in MCF7-shWISP2 cells, with the exception of KLF4, which was strongly overexpressed in these cells as compared with the MCF7 parental and -shScrambled cells (Figure 3a). These results suggest that activation of TGF-β signaling in the EMT MCF7-shWISP2 cells confers the stem cell-like properties to these cells through the positive regulation of KLF4 expression as demonstrated by an increased exclusion of the Hoechst dye (Supplementary Figure 2) and an exhibition of a CD44High/CD24Low profile (data not shown).
Selective induction of KLF4 following WISP2 silencing and its silencing alleviates MCF7-shWISP2 resistance to CTLs. (a) RT–qPCR showing relative messenger RNA (mRNA) expression of stemness markers. Values were normalized to 18s rRNA levels. (b) CTL-mediated cytotoxicity toward tumor cells at different effector: target (E:T) ratios. Western blot analysis of KLF4 expression in untransfected cells and in cells transfected with either KLF4 or luciferase siRNAs. Significant P-values are indicated by asterisks (*P=0.03; **P=0.003; ***P<0.001).
To determine the putative role of this transcription factor in the regulation of MCF7-shWISP2 cell susceptibility to CTL-mediated lysis, we explored the functional consequences of KLF4 silencing using specific small interfering RNA (siRNA). MCF7-shWISP2 cells were silenced for KLF4 and subsequently used as target cells in a cytotoxic assay. As shown in Figure 3b, silencing of KLF4 significantly restored the susceptibility of MCF7-shWISP2 cells to CTL clone-mediated lysis. These results indicate that KLF4 has an important role in the regulation of mesenchymal cell susceptibility to CTL-mediated lysis.
Inhibition of TGF-β signaling reverses EMT phenotype, KLF-4 expression and restores susceptibility to CTL-mediated lysis
To determine whether inhibition of TGF-β signaling, restores target cell phenotype and susceptibility to CTLs, MCF7-shWISP2 cells were treated with A83-01, a small molecule that selectively inhibits ALK4, ALK5 and ALK7 receptors. Cells were then tested for EMT phenotype, KLF4 expression and killing by the CTL clone. As shown in Figure 4a, such treatment resulted in Smad2 phosphorylation inhibition, E-cadherin expression and in a decrease in the mesenchymal marker, Twist expression. Immunofluorescence staining showed a partial phenotype reversion using F-actin, E-cadherin and ZO-1 EMT markers (Figure 4b). More importantly, treatment with A83-01 partially restored CTL-mediated lysis of MCF7-shWISP2 cells (Figure 4c) that correlates with a marked inhibiton of KLF4 transcription inhibition (Figure 3d).
The impact of TGF-β signaling pathway inhibition on mesenchymal phenotype, CTL-mediated lysis and KLF4 expression. (a) Western blot analysis of TGF-β signaling pathway proteins expression and EMT markers. (b) Immunofluorescence staining of actin and epithelial markers (E-cadherin and ZO-1). Nuclei were stained with 4′,6-diamidino-2-phenylindole. (c) Cytotoxicity was determined by a conventional 4 h 51Cr release assay at different ratios. Bars indicate s.d. (d) RT–qPCR showing relative mRNA expression of KLF4. Values were normalized to 18s rRNA levels (*P<0.05; and ***P< 0.0005).
KLF4 coexpresses with EMT markers and TGF-β signaling pathway proteins
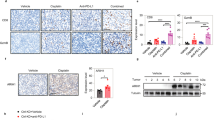
We identified in vitro that WISP2 silencing induced activation of the TGF-β/Smad signaling pathway to regulate KLF4 expression. Thus, we next asked whether EMT markers, TGF-β pathway proteins and KLF4 were co-expressed in human breast carcinoma tissue samples of the basal-like subtype using immunohistochemistry. For this purpose, 20 patient biopsies were used. We found that the expression of EMT and TGF-β signaling markers correlated with KLF4 expression in these samples (Figure 5). As depicted in Figure 5, Twist, TGF-β and SMAD2/3 proteins and a loss of E-cadherin expression were localized in KLF4-positive areas of the tumors. These findings illustrate that EMT/TGF-β-signaling markers are co-expressed with KLF4 in human invasive basal breast carcinoma.
Coexpression of EMT markers (Twist and E-cadherin), TGF-β signaling pathway proteins (TGF-β and SMAD2/3) and KLF4 in human breast cancer biopsies. Serial sections from human breast cancer biopsies were stained using immunohistochemistry. Right panel shows an enlarged view of the boxed areas in the left panel at × 250 magnification.
TGF-β-mediated inhibition of target cell susceptibility to specific lysis through regulation of miR-7 expression
To gain further insight into how TGF-β signaling regulates the expression of KLF4, we performed microRNA (miRNA) profiling using the A83-01 inhibitor of TGF-β signaling and assessed the expression of 59 miRNAs in MCF7-shWISP2 and -shWISP2+A83-01 cells (Figure 6A). Profiling in MCF7-shWISP2 cells yielded a list of 26 (44%) up- and 29 (49%) downregulated miRNAs with A83-01 treatment as compared with untreated MCF7-shWISP2 cells, thus displaying an activation in TGF-β signaling. Only four miRNAs (6.7%; indicated as white boxes) in MCF7-shWISP2 cells were unaffected by the inhibitor. Similar profiling was next performed in untreated and A83-01-treated MCF7-shWISP2 cells displaying inhibited TGF-β signaling. Interestingly, we showed that the number of up- and downregulated miRNAs markedly dropped to only two up- and two downregulated miRNAs (Figure 6B). These data indicate that the activation of TGF-β signaling in MCF7-shWISP2 cells regulates some miRNAs involved in the regulation of several signaling pathways. Having demonstrated that KLF4 has a critical role in MCF7-shWISP2 cells, we focused our attention on miRNAs that target this gene. In silico analysis using the miRDB database revealed that KLF4 is predicted to be targeted by 47 miRNAs. Among these 47 miRNAs, we identified four candidates (miR-7-5p, miR-429, miR-200b-3p and miR-200c-3p) whose expression was inversely correlated with that of KLF4 in shWISP2-expressing cells (Figure 6C; indicated by the arrowhead in Figure 6A). The regulated expression of these miRNAs was validated by RT–PCR in MCF7-shScrambled, MCF7-shWISP2 and A83-01-treated MCF7-shWISP2 cells (Figure 6D). This result further indicates that the impairment of CTL-mediated lysis in MCF7-shWISP2 cells is most likely related to the TGF-β-dependent inhibition of miR-7-5p, miR-429, miR-200b-3p and miR-200c-3p expression involved in targeting KLF4. To investigate which miRNA is predominantly involved in this event, we restored the expression of each miRNA in MCF7-shWISP2 cells using miRNAs precursors (Figure 6F (a)). The cytotoxicity assay showed that only restoration of miR-7-5p was able to significantly restore CTL-mediated lysis (Figure 6F (b)). The regulation of KLF4 expression by miR-7 is confirmed by western blot analyses (Figure 6E). These results indicate that the TGF-β-mediated inhibition of mesenchymal cell susceptibility to T-cell-mediated lysis involves miR-7 inhibition.
The impact of TGF-β-induced EMT on miRNA expression. (A) Heat map of miRNAs regulated by TGF-β signaling pathway in cell lines. Red signifies upregulation, whereas green signifies downregulation. (B) Number of miRNAs up- and downregulated with or without A83-01 (A83) treatment. (C) Relationship between KLF4 expression and miR-7-5p, miR-429 miR-200b-3p and miR-200c-3p expression (indicated with bold arrows in A). (D) miRNAs expression was monitored by TaqMan qRT–PCR. Expression levels of RNU44 were used as an endogenous control. (E) (a) Control of Pre-miRNAs-precursors transfection quality by TaqMan qRT–PCR. Expression levels of RNU44 were used as an endogenous control. (b) CTL-mediated lysis of tumor cells at different effector:target (E:T) ratios. Bars indicate mean and errors indicate the s.d. Significant P-values are indicated by asterisks (*P=0.03; **P=0.003; ***P=0.001). (F) Western blot analysis of KLF4 expression following transfection with miR-7 precursor.
Discussion
An increasing accumulation of data supports the notion that the tumor stroma has a crucial role in controlling immune protection and contains many overlapping mechanisms to maintain tumor functional disorder and evasion from antigen-specific immunotherapy. In addition, the complexity of the tumor ecosystem in its deviation of the functions of tumor-infiltrating cells and in its activation of tumor resistance mechanisms is well established. In this context, the aim of this study was to explore the role of WISP2 in the acquisition of cell plasticity and determine whether it interferes with the acquisition of tumor cell resistance to CTL-mediated cytotoxicity, using in vitro cultured breast cancer cells as a model. We first employed new approaches, based on the use of Single Sample Geneset Enrichment Analysis and EMT scoring, to illustrate that WISP2 silencing in breast cancer cells activated TGF-β signaling and promoted EMT.
TGF-β, an important regulator of immune functions, suppresses T-cell-mediated anti-tumor immunity by directly inhibiting the differentiation and functions of various effector T lymphocytes, such as Th1 cells and cytotoxic CD8+ T lymphocytes.20 In addition to its direct immune suppression, we here show that the activation of TGF-β signaling, following WISP2 silencing in tumor cells, exerts a dual effect by promoting the EMT phenotype and subsequently impairing CTL effector functions. This finding is in support of our previous studies that showed that Snail-induced EMT confers resistance of target cells to specific CTL activity.13,14 As Ag recognition by tumor-specific CD8+ T cells strictly correlates with the efficiency of tumor recognition, we sought to gain more insight into WISP2 knockdown-induced impairment of CTL activity by addressing whether WISP2 interferes with the cross-talk between effector and target cells and, subsequently, target recognition. Our data indicate that WISP2 silencing has an inhibitory effect on TAP1, TAP2 and HLA-A2 protein expression levels, which ultimately resulted in an attenuation of CTL clone reactivity, as revealed by its capacity to secrete TNF-β. Using MDA-MB-231 cell line that displays no WISP2 expression or secretion. We showed that these cells had a significant decrease in susceptibility to CTL lysis as compared with the MCF7 cell line, confirming the effect of WISP2 silencing on CTL-mediated killing (Supplementary Figure 1A). However, although no difference in terms of HLA-A2 molecule expression in MDA-MB-231 cells was observed (Supplementary Figure 1B), a significant decrease in TAP1 and TAP2 protein expression was detected (Supplementary Figure 1C). This suggests that the decrease in CTL activity toward MCF7-shWISP2 and MDA-MB-231 cell lines involves at least in part TAP1 and TAP2 molecule expression.
To elucidate the mechanism by which WISP2 could regulate HLA-A2, TAP1 and TAP2 expression, we performed qRT–PCR analyses to investigate whether HLA-A2, TAP1 and TAP2 are regulated at the transcriptional level by WISP2. The results shown in Supplementary Figure 3 demonstrate that HLA-A2 and TAP2 decreased in shWISP2 cells, suggesting a transcriptional regulation of those genes following WISP2 inhibition. With respect to TAP1 inhibition, no decrease in its mRNA expression was observed in shWISP2 cells. Owing to the scope of this report, we believe that this topic should be the subject of our future study.
To determine whether impairment of T-cell-mediated lysis correlated with defects in the recognition of shWISP2 cells, we examined conjugate formation between CTL and MCF7 cells or shWISP2-treated and shScrambled-treated cells by confocal microscopy. The results shown in Supplementary Figure 4A indicate that MCF7 parental and shScrambled cells were able to similarly form stable conjugates with the CTL and could induce T-cell activation, as shown by an increase in tyrosine phosphorylation at the cell contact area (Supplementary Figure 4A). However, a significant decrease in phosphotyrosine staining was detected when the CTL clone was cultured with shWISP2 cells (Supplementary Figure 4A). The decrease in phosphotyrosine accumulation at the contact zone formed between shWISP2 cells and CD8+ T cells revealed an alteration in the signaling events occurring at the immune synapse. When reported as a percentage of active conjugates, we observed a significant decrease in conjugates with phosphotyrosine accumulation between shWISP2 cells and CD8+ T lymphocytes as compared with those observed between parental MCF7 or shScrambled cell lines and CTLs (Supplementary Figure 4B). These results emphasize that WISP2 has implications in tumor recognition by immune cells, as well as in tumor cell killing, by shaping its plasticity following EMT phenotype acquisition.
A relationship between EMT and breast cancer stem cells (CSC) has emerged, with evidence that the progression of a mammary epithelial cell through an EMT generates cells with properties of breast CSCs.21 In addition, recent studies have provided strong evidence for the critical role of TGF-β signaling in regulating the dynamics of cell populations within malignant breast tumors, achieved by favouring the acquisition of CSC-like properties.22 Indeed, one report shows that the acquisition of stem cell-like properties may be a function of the stromal microenvironment.23 In this regard, we asked whether WISP2 silencing (a) induced the inhibition of target cell susceptibility to specific lysis, (b) interferes with the regulation of transcription factors associated with the self-renewal potential of CSCs and (c) is able to endow tumor cells with CSC-like features through regulation of miRNA expression.
Our data clearly show a selective induction of KLF4 in WISP2-deficient cells. Several studies have indicated that the poor clinical outcomes of vaccination are primarily caused by mechanisms of immune tolerance, especially within the tumor microenvironment. Here, we report that activation of TGF-β signaling in tumor target cells following WISP2 inhibition drives the evolution of these cells toward immune resistance by acquiring a stem-like phenotype that alters the CTL response.
Although KLF4 was found to be highly expressed in >70% of breast cancers and functions as an oncogene, the exact mechanism by which KLF4 enhances tumorigenesis and breast cancer cell immunosurveillance evasion remains unknown. Its potent oncogenic role in mammary tumorigenesis likely occurs by maintaining stem cell-like features and by promoting cell migration and invasion.24 The current studies clearly point to a direct and critical role of TGF-β-dependent induction of KLF4 in the impairment of CTL-mediated tumor cell lysis. Indeed, CSCs have been shown to resist cell death induced by apoptotic and chemo- or radiotherapeutic agents.25,26 Here, we provide evidence indicating that KLF4 induced in target cells is an additional stemness transcription factor having a crucial role in the regulation of target cell susceptibility to specific lysis. With respect to the regulation of KLF4 expression, in agreement with our finding, Okuda et al.24 found that miR-7 attenuates CSC invasion and self-renewal by modulating KLF4 expression. However, Hu and Wan27, using Mv1Lu (Mink lung) epithelial cells and HEK293T (human embryonic kidney) cells, have demonstrated that KLF4 is profoundly degraded in response to TGF-β signaling by a mechanism involving the ubiquitin-proteasome pathway. This fits with the report by Liu et al.28 using mice cell lines isolated from prostate tumors indicating that, following TGF-β treatment, there is a rapid loss of KLF4 RNA and a significant reduction in KLF4 protein levels. Using rat vascular smooth muscle cells, Zhang et al.29 have reported that treatment of these cells with TGF-β resulted in an increase of KLF4 protein. These findings surrounding the proteolytic regulation of KLF4 by TGF-β signaling support the notion that TGF-β-mediated KLF4 regulation is highly cell-context dependent.
KLF4 is known to regulate proliferation, differentiation and apoptosis.30 Other findings support a crucial role for this transcription factor in the maintenance of genetic stability by modulating the DNA damage response and repair processes.31 It is tempting to speculate that, in response to microenvironmental cues and their signaling, tumor cells can alter their phenotype and, consequently, modulate tumor heterogeneity and anti-tumor immune responses. Indeed, TGF-β is a critical component of the tumor microenvironment. MCF7-shWISP2 cells were treated with A83-01, a small-molecule inhibitor of TGF-β receptor type I kinase, to ascertain whether inhibiting TGF-β signaling could restore target cell phenotypes and their susceptibility to CTLs. Indeed, we showed that such treatment resulted in an inhibition of Smad2 phosphorylation (Figure 4a), and a partial reversal of the EMT phenotype (Figures 4a and b). Moreover, TGF-β inhibition in part restored CTL-mediated lysis of MCF7-shWISP2 cells (Figure 4c), which correlated with a marked inhibition in KLF4 transcription (Figure 4d). In addition, western blot analyses revealed an inhibition of KLF4 expression following Smad2 silencing in MCF7-shWISP2 cells (Supplementary Figure 5). These observations suggest that TGF-β antagonists enhance anti-tumor immune responses by blocking signaling and the subsequent targeting of multiple cellular components. It should be emphasized that not only the systemic neutralization but also the selective blockade of TGF-β signaling may be effective in regulating anti-tumor responses, as A83-01 was effective in suppressing melanoma bone metastases.32
miRNAs have been reported to regulate transcription factors associated with stemness. In this regard, miRNA-145 has been reported to regulate OCT4, SOX2 and KLF4 and represses pluripotency in human embryonic stem cells.33 To advance our understanding of how TGF-β signaling regulates the expression of KLF4, we performed miRNAs profiling in untreated and A83-01-treated MCF7-shWISP2 cells. The activation of TGF-β signaling in MCF7-shWISP2 cells was found to regulate some miRNAs involved in the control of several signaling pathways. However, in our experimental system, only the restoration of miR-7-5p could significantly restore CTL-mediated lysis. It has been reported that miR-7-5p expression is reduced in metastatic melanoma-derived cell lines compared with primary melanoma cells and that, when ectopically expressed, miR-7-5p significantly inhibits melanoma cell migration and invasion.34 In addition, it was suggested that miR-7-5p may represent a novel tumor suppressor miRNA, acting at least in part via its inhibition of oncogenic Akt signaling.35 miR-7-5p may therefore represent a novel therapeutic approach to prevent or limit tumor metastasis. Moreover, a very recent study has shown that miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4 expression.24 Our data point to a novel function of miR-7-5p-regulated KLF4 in the control of mesenchymal tumor cell susceptibility to specific lysis. Thus, our study suggests the existence of a new relationship between WISP2, KLF4, miR-7-5p and resistance to CTL-mediated lysis.
Taken together, our results suggest that WISP2 may orchestrate a dynamic cross-talk between the killer and target cell-signaling pathways that have a key role in the control of tumor target sensitivity to CTL-induced cell death. A summary of the study findings is presented in Figure 7.
A schematic diagram summarizing the findings of this study. WISP2 silencing triggered on one hand, activation of TGF-β signaling-induced EMT and downregulation of miR-7 expression that controls KLF-4, and on the other hand, downregulation of HLA-A2, TAP1 and TAP2 molecule expression. Combined, these molecular events regulation result in a decrease in tumor cell susceptibility to CTL-mediated lysis.
Materials and methods
Plasmids
The entire open-reading frame of WISP-2/CCN5 complementary DNA was inserted into the pCEP4-Flag vector (Invitrogen, Illkirch, France), herein referred to as pCEP4-Flag-WISP-2. siRNA oligonucleotides for WISP-2/CCN5 were designed using the Target Finder program (Ambion, Austin, TX, USA). The following sequences were used to construct short hairpin RNA interference vectors in pSilencer (Ambion): shWISP2, 5′-AAGGTGCGTACCCAGCTGTG-3′, and shScrambled, 5′-AGTACGTGTACAGGCGCCGT-3′, leading to the pSilencer/shWISP2 (MCF7-shWISP2) and the pSilencer/shScrambled vector (MCF7-shScrambled), respectively.
T-cell clone, tumor cell lines and culture conditions
The T-cell-receptor Vβ13.6+ Heu33 CTL clone was derived from the tumor of a lung cancer patient, as previously described.18 MCF-7 and derivatives were grown in Dulbecco's modified Eagle's medium/F12 medium supplemented with 10% heat-inactivated fetal calf serum (Gibco-BRL, Gaithersburg, MD, USA), 1 mM sodium pyruvate (Life Technologies, Carlsbad, CA, USA) and 1% penicillin-streptomycin (Life Technologies) at 37 °C in a humidified atmosphere containing 5% CO2. For MCF7-shWISP2 and MCF7-shScrambled, 100 μg/ml of Hygromycin-B (Life Technologies) was added to the complete medium.
Cell morphology, actin cytoskeleton and EMT markers staining
Cell morphology images were acquired using a × 20 objective lens and phase contrast microscopy (Leica Microsystems, Wetzlar, Germany). Actin filaments were stained by Alexa Fluor 488-coupled Phalloidin. EMT markers were stained with the indicated antibodies (Abs) and nuclei with 4′,6-diamidino-2-phenylindole, dihydrochloride (Life Technologies). Cells were cultured in IBIDI chambers (IBIDI, Biovalley, France) and fixed with 3% paraformaldehyde for 20 min at room temperature. Cells were then permeabilised with 0.4% Triton X-100, and unspecific sites were blocked with phosphate-buffered saline-10% fetal bovine serum for 15 min at room temperature before staining with the indicated Abs. Cells were analyzed with a Zeiss laser scanning confocal microscope, LSM-510 Meta (Carl Zeiss, Oberkochen, Germany) and processed by LSM Image Examiner software (Carl Zeiss).
Western blotting
Adherent cells were lysed on the plate with lysis buffer (62.5 mM Tris-HCl (pH 6.8), 2% w/v SDS, 10% glycerol, 1 mM sodium orthovanadate, 2 mM phenylmethylsulfonyl fluoride, 25 μM leupeptin, 5 mM benzamidine, 1 μM pepstatin and 25 μM aprotinin). Cells lysates were resolved by SDS–polyacrylamide gel electrophoresis (30 μg/well) and transferred onto nitrocellulose membranes. After incubation in blocking buffer, the membranes were probed overnight at 4 °C with the primary Abs. The labeling was visualized using peroxidase-conjugated secondary Abs and with an ECL kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Primary Abs against SNAI1 and E-cadherin were from Cell Signalling (Beverly, MA). Abs against SNAI2 and Twist were from Abcam (Cambridge, MA, USA). Abs against Vimentin and N-cadherin were from Santa Cruz Biotechnology (Dallas, TX, USA). Abs against TAP1 and TAP2 were from E Wiertz (Utrecht, the Netherlands).
Data pre-processing of the breast cancer cell line panel
Two large breast cancer cell line data sets were established using the Affymetrix (Santa Clara, CA, USA) U133A or U133plus2 platform: E-TABM-157 (n=51 samples corresponding to 51 cell lines), and GSE15026 (n=30 samples corresponding to 19 cell lines) were downloaded from Array Express and Gene Expression Omnibus, respectively. Robust multichip average normalization was performed for each data set. The normalized data were combined with the data set from the present study (n=6 samples from the parental MCF-7 cells, shWISP2-transfected cells and shScrambled-transfected cells), and subsequently standardized using ComBat36 to remove the batch effect. The standardized data yielded a data set of 89 samples derived from 73 cell lines.
Estimation of EMT score
An EMT scoring method was developed using ovarian carcinoma cell line expression profiling. The first step was to establish an EMT signature comparing profiles of E-cadherin/cadherin-1 with CDH-2-expressing cell lines using a binary regression method (BinReg).3 In the second step, the BinReg ovarian cancer EMT signature was applied to breast cancer cell lines to predict their EMT status. In the third step, the top 25% (~20 samples) with the highest probabilities for an epithelial or mesenchymal phenotype were used to obtain epithelial- or mesenchymal-specific gene lists for the breast cancer cell lines using significance analysis of microarray q-value of 0 and receiver operating characteristic value of 0.85. In the fourth step, Single-sample geneset enrichment analysis15 was employed to compute the enrichment score of a cell line based on the expression of the breast cancer cell line-specific epithelial or mesenchymal signature genes. The EMT score is defined as the normalized subtraction of the mesenchymal from epithelial enrichment scores. The EMT score is a precise estimate of the cell line’s status as an epithelial or mesenchymal phenotype. A higher or lower EMT score indicates that the cell line exhibits a more mesenchymal or epithelial phenotype, respectively.
Cytotoxicity experiments
The cytotoxic activity of the CTL clone was measured by a conventional 4 h 51Cr release assay. Several ratios of effector:target (E:T) were used on 1000 target cells per well. Supernatants were then transferred to LumaPlate-96 wells (PerkinElmer, Waltham, MA, USA), dried and counted on a Packard TopCount NXT (PerkinElmer, Santa Clara, CA, USA). Percent specific cytotoxicity was calculated conventionally.
Surface expression of HLA-A2
HLA-A2 molecule expression on tumor cell lines was quantified by immunofluorescence using MA2.1 monoclonal Ab for HLA-A2 (produced in the laboratory).
TNF-β production assay
TNF-β release was detected by measuring the cytotoxicity of the culture supernatants on the TNF-sensitive WEHI-164c13 cells with an MTT (bromure de 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium) colorimetric assay, as previously described.38
RNA isolation and SYBR Green qRT–PCR
Total RNA was extracted from the samples using TRIzol (Invitrogen). A total of 1 μg RNA was converted into complementary DNA using the Taqman reverse transcription reagent from Applied Biosystems (Life Technologies, Foster City, CA, USA) and mRNA levels were quantified by SYBR Green qPCR (Applied Biosystems). Relative expression was calculated by using the comparative CT method (2-ΔCT).
Flow cytometry and Hoechst assay
Cells were harvested with 0.025% EDTA and double-stained with CD44 (PE) and CD24 (allophycocyanin) monoclonal Abs, both from Miltenyi Biotec (Auburn, CA, USA). Labeled cells were analyzed on a BD FACScalibur (BD Biosciences, Franklin Lakes, NJ, USA).
Exclusion of Hoechst 33342 dye assay was performed using Hoechst 33342 from Sigma Aldrich (St Louis, MO, USA) following the manufacturer's recommendations. Labeled cells were analyzed on BD LSR Flow Cytometer (BD Biosciences).
A83-01 treatment
Cells were treated with A83-01 (10 μM), a small-molecule inhibitor of TGF-β receptor type I kinase activity (Tocris Bioscience, Bristol, UK), or dimethyl sulfoxide for 72 h.
siRNA transfection
The siRNA used was purchased from Sigma Aldrich. Luciferase (Luc) siRNA was used as a negative control. Subconfluent cells were transfected with siRNA in Opti-MEM, using RNAiMax reagent (Invitrogen) according to the manufacturer’s instructions. The silencing of KLF4 was assessed by western blotting 48 h after transfection using appropriate Abs (Cell Signalling).
Immunohistochemical staining for E-cadherin, Twist, TGF-β, SMAD2/3 and KLF4 expression
Immunohistochemistry on human breast cancer serial sections was performed as previously described.39 The samples were incubated with monoclonal Abs against E-cadherin (Cell Signalling), Twist (Abcam), TGF-β (Abcam), SMAD2/3 (Abcam) and KLF4 (Sigma Aldrich). The signal was revealed with the DAB HistoMouse-Max kit (Zymed Laboratories Inc., South San Francisco, CA, USA).
MicroRNA (miR) isolation and detection and microRNA microarray experiment
TRIzol (Invitrogen) was used to extract miRNAs. miRNA microarray analysis was conducted using Agilent human miRNA microarray (Agilent Technologies, Santa Clara, CA, USA). Rosetta resolver software (Rosetta Biosoftware, Seattle, WA, USA) was used for the analysis. DNase I-treated total RNA (8 ng) was subjected to quantitative RT–PCR analysis using TaqMan miRNA Reverse Transcription Kit (Applied Biosystems). miRNAs were detected and quantified using specific miRNA primers from Ambion, and the expression levels of mature miRNAs were evaluated using the comparative Ct method (ΔΔCt). Transcript levels of RNU44 were used as an endogenous control.
Statistical analyses
Data were analyzed with GraphPad Prism (GraphPad Software, La Jolla, CA, USA). A Student’s t-test was used for single comparisons. Data were considered statistically significant when P<0.05.
References
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3: 991–998.
Anichini A, Molla A, Mortarini R, Tragni G, Bersani I, Di Nicola M et al. An expanded peripheral T cell population to a cytotoxic T lymphocyte (Ctl)-defined, melanocyte-specific antigen in metastatic melanoma patients impacts on generation of peptide-specific ctls but does not overcome tumor escape from immune surveillance in metastatic lesions. J Exp Med 1999; 190: 651–667.
Chouaib S Integrating the quality of the cytotoxic response and tumor susceptibility into the design of protective vaccines in tumor immunotherapy. J Clin Invest 2003; 111: 595–597.
Hamai A, Benlalam H, Meslin F, Hasmim M, Carre T, Akalay I et al. Immune surveillance of human cancer: if the cytotoxic T-lymphocytes play the music, does the tumoral system call the tune? Tissue Antigens 2010; 75: 1–8.
Holzel M, Bovier A, Tuting T . Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nat Rev Cancer 2013; 13: 365–376.
Yang J, Weinberg RA Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008; 14: 818–829.
Banerjee S, Saxena N, Sengupta K, Tawfik O, Mayo MS, Banerjee SK Wisp-2 gene in human breast cancer: estrogen and progesterone inducible expression and regulation of tumor cell proliferation. Neoplasia 2003; 5: 63–73.
Fritah A, Saucier C, De Wever O, Bracke M, Bieche I, Lidereau R et al. Role of Wisp-2/Ccn5 in the maintenance of a differentiated and noninvasive phenotype in human breast cancer cells. Mol Cell Biol 2008; 28: 1114–1123.
Banerjee S, Dhar G, Haque I, Kambhampati S, Mehta S, Sengupta K et al. Ccn5/Wisp-2 expression in breast adenocarcinoma is associated with less frequent progression of the disease and suppresses the invasive phenotypes of tumor cells. Cancer Res 2008; 68: 7606–7612.
Sabbah M, Prunier C, Ferrand N, Megalophonos V, Lambein K, De Wever O et al. Ccn5, a novel transcriptional repressor of the transforming growth factor beta signaling pathway. Mol Cell Biol 2011; 31: 1459–1469.
Dhar G, Banerjee S, Dhar K, Tawfik O, Mayo MS, Vanveldhuizen PJ et al. Gain of oncogenic function of P53 mutants induces invasive phenotypes in human breast cancer cells by silencing Ccn5/Wisp-2. Cancer Res 2008; 68: 4580–4587.
Frewer KA, Sanders AJ, Owen S, Frewer NC, Hargest R, Jiang WG A role for Wisp2 in colorectal cancer cell invasion and motility. Cancer Genomics Proteomics 2013; 10: 187–196.
Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res 2013; 73: 2418–2427.
Akalay I, Janji B, Hasmim M, Noman MZ, Thiery JP, Mami-Chouaib F et al. EMT impairs breast carcinoma cell susceptibility to ctl-mediated lysis through autophagy induction. Autophagy 2013; 9: 1104–1106.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in Pdgfra, Idh1, Egfr, and Nf1. Cancer Cell 2010; 17: 98–110.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006; 10: 515–527.
Dorothee G, Echchakir H, Le Maux Chansac B, Vergnon I, El Hage F, Moretta A et al. Functional and molecular characterization of a Kir3dl2/P140 expressing tumor-specific cytotoxic T lymphocyte clone infiltrating a human lung carcinoma. Oncogene 2003; 22: 7192–7198.
Hasmim M, Noman MZ, Lauriol J, Benlalam H, Mallavialle A, Rosselli F et al. Hypoxia-dependent inhibition of tumor cell susceptibility to Ctl-mediated lysis involves nanog induction in target cells. J Immunol 2011; 187: 4031–4039.
Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006; 24: 99–146.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715.
Liu Z, Bandyopadhyay A, Nichols RW, Wang L, Hinck AP, Wang S et al. Blockade of autocrine Tgf-beta signaling inhibits stem cell phenotype, survival, and metastasis of murine breast cancer cells. J Stem Cell Res Ther 2012; 2: 1–8.
Reiman JM, Knutson KL, Radisky DC Immune promotion of epithelial-mesenchymal transition and generation of breast cancer stem cells. Cancer Res 2010; 70: 3005–3008.
Okuda H, Xing F, Pandey PR, Sharma S, Watabe M, Pai SK et al. Mir-7 suppresses brain metastasis of breast cancer stem-like cells by modulating Klf4. Cancer Res 2013; 73: 1434–1444.
Rebucci M, Michiels C Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol 2013; 85: 1219–1226.
Shekhani MT, Jayanthy AS, Maddodi N, Setaluri V Cancer stem cells and tumor transdifferentiation: implications for novel therapeutic strategies. Am J Stem Cells 2013; 2: 52–61.
Hu D, Wan Y Regulation of Kruppel-like factor 4 by the anaphase promoting complex pathway is involved in Tgf-beta signaling. J Biol Chem 2011; 286: 6890–6901.
Liu YN, Abou-Kheir W, Yin JJ, Fang L, Hynes P, Casey O et al. Critical and reciprocal regulation of Klf4 and slug in transforming growth factor beta-initiated prostate cancer epithelial-mesenchymal transition. Mol Cell Biol 2012; 32: 941–953.
Zhang XH, Zheng B, Gu C, Fu JR, Wen JK . TGF-beta1 downregulates AT1 receptor expression via PKC-delta-mediated Sp1 dissociation from KLF4 and Smad-mediated PPAR-gamma association with KLF4. Arterioscler Thromb Vasc Biol 2012; 32: 1015–1023.
Yang Y, Goldstein BG, Chao HH, Katz JP . Klf4 and Klf5 regulate proliferation, apoptosis and invasion in esophageal cancer cells. Cancer Biol Ther 2005; 4: 1216–1221.
El-Karim EA, Hagos EG, Ghaleb AM, Yu B, Yang VW Kruppel-like factor 4 regulates genetic stability in mouse embryonic fibroblasts. Mol Cancer 2013; 12: 89.
Mohammad KS, Javelaud D, Fournier PG, Niewolna M, McKenna CR, Peng XH et al. Tgf-Beta-Ri kinase inhibitor Sd-208 reduces the development and progression of melanoma bone metastases. Cancer Res 2011; 71: 175–184.
Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS Microrna-145 regulates Oct4, Sox2, and Klf4 and represses pluripotency in human embryonic stem cells. Cell 2009; 137: 647–658.
Giles KM, Brown RA, Epis MR, Kalinowski FC, Leedman PJ Mirna-7-5p inhibits melanoma cell migration and invasion. Biochem Biophys Res Commun 2013; 430: 706–710.
Fang Y, Xue JL, Shen Q, Chen J, Tian L Microrna-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology 2012; 55: 1852–1862.
Johnson WE, Li C, Rabinovic A . Adjusting batch effects in microarray expression data using empirical bayes methods. Biostatistics 2007; 8: 118–127.
Gatza ML, Lucas JE, Barry WT, Kim JW, Wang Q, Crawford MD et al. A pathway-based classification of human breast cancer. Proc Natl Acad Sci USA 2010; 107: 6994–6999.
El Hage F, Stroobant V, Vergnon I, Baurain JF, Echchakir H, Lazar V et al. Preprocalcitonin signal peptide generates a cytotoxic T lymphocyte-defined tumor epitope processed by a proteasome-independent pathway. Proc Natl Acad Sci USA 2008; 105: 10119–10124.
Magnon C, Opolon P, Ricard M, Connault E, Ardouin P, Galaup A et al. Radiation and inhibition of angiogenesis by canstatin synergize to induce Hif-1alpha-mediated tumor apoptotic switch. J Clin Invest 2007; 117: 1844–1855.
Acknowledgements
This work was supported by grants from INSERM, la Ligue Nationale Contre le Cancer, l’Association de Recherche sur le Cancer, A*STAR Institute of Molecular Cell Biology, Cancer Science Institute National University of Singapore core grants, CRP-Santé and Fondation Cancer, Luxembourg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Akalay, I., Tan, T., Kumar, P. et al. Targeting WNT1-inducible signaling pathway protein 2 alters human breast cancer cell susceptibility to specific lysis through regulation of KLF-4 and miR-7 expression. Oncogene 34, 2261–2271 (2015). https://doi.org/10.1038/onc.2014.151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.151
This article is cited by
-
The loss of B7-H4 expression in breast cancer cells escaping from T cell cytotoxicity contributes to epithelial-to-mesenchymal transition
Breast Cancer Research (2023)
-
Harnessing epithelial-mesenchymal plasticity to boost cancer immunotherapy
Cellular & Molecular Immunology (2023)
-
EMT-induced immune evasion: connecting the dots from mechanisms to therapy
Clinical and Experimental Medicine (2023)
-
Differential expression profile of plasma exosomal microRNAs in acute type A aortic dissection with acute lung injury
Scientific Reports (2022)
-
Identification of the prognostic value of a 2-gene signature of the WNT gene family in UCEC using bioinformatics and real-world data
Cancer Cell International (2021)