Abstract
The mean-square radii of gyration <S2> of two polystyrene (PS) samples with weight-average molar masses Mw of 2.18 × 104 and 3.88 × 104 in toluene and 2-butanone and of a cyclic amylose tris(phenylcarbamate) (cATPC) with a Mw of 4.73 × 104 in tetrahydrofuran were determined by synchrotron radiation small-angle X-ray scattering measurements over a wide range of temperatures from −77 °C to 70 °C. Both PS and cATPC are sufficiently soluble to enable SAXS measurements even at −77 °C in the solvents used. The <S2> of cATPC does not depend on temperature over the range investigated here. This result may be reasonable for such rigid ring polymers. In contrast, the radii of PS depend on temperature to a significant degree, whereas the second virial coefficient is mostly temperature independent. The resulting characteristic ratio C∞ for PS in toluene decreases monotonically with increasing temperature, as predicted both by the rotational isomeric state (RIS) and by (helical) wormlike chain models. However, C∞ in 2-butanone exhibits a minimum ∼10 °C and increases with increasing temperature, suggesting that the RIS energy parameters should be affected by the intermolecular interactions between the polymer and solvent.
Similar content being viewed by others
Introduction
The unperturbed dimensions of polymers in solution are discussed in terms of the rotational isomeric state (RIS) model1 and/or the (helical) wormlike chain model.2, 3, 4 Both models predict that the unperturbed dimensions depend on temperature. The chain dimensions were observed to depend on temperature for some flexible polymers, for example, polystyrene (PS),5, 6 poly(α-methylstyrene),6, 7 1,4-polybutadiene7 and syndiotactic poly(methylmethacrylate)8 from near room temperature to ∼60 °C. Other than this temperature range, Hong et al.9 measured light scattering for PS up to 140 °C to investigate excluded-volume effects. Furthermore, the intrinsic viscosities of poly(n-hexylisocyanate),10 polydialkylsilanes,11, 12 polyfluorene13 and cellulose tris(phenylcarbamate)14 were also determined at various temperatures to determine the variation in chain stiffness with temperature, which is directly related to the unperturbed chain dimensions, but −27 °C was the lowest temperature studied here. Although this temperature is still much higher than the melting points for some major organic solvents, that is,∼−100 °C, several scattering experiments have been conducted at lower temperatures such as PS in carbon disulfide15, 16, 17 and cellulose triacetate in methyl acetate18 to investigate gel formation. Determining the polymer dimensions in solution over a wider range of temperature between the melting and boiling points should be helpful in understanding the temperature-dependent conformation of polymer chains in more detail. However, conventional static light scattering methods cannot be used at low temperatures due to water condensation because most light scattering instruments have large thermostated baths with refractive index matching media, such as toluene, xylene and specific silicone oils, to avoid stray light. A thin capillary tube can be used for small-angle X-ray scattering19 because the refractive indices of quartz and organic solvents are substantially close to unity.
Therefore, we performed synchrotron radiation small-angle X-ray scattering (SAXS) measurements of PS in toluene and in 2-butanone (MEK) at temperatures between −77 °C and 70 °C to investigate the temperature coefficient of unperturbed chain dimensions. The recently investigated cyclic amylose tris(phenylcarbamate) (cATPC)20 that acts as rigid ring in solution was also measured in tetrahydrofuran (THF) to test the apparatus. As mentioned in our recent paper, cATPC50K has substantially the same <S2>z as the rigid ring limit.20 Thus, the dimensional properties of cATPC50K should not exhibit any temperature dependence in THF. Note that all three systems are good solvents near room temperature.6, 20, 21
Experimental procedure
Samples and solvents
The two previously investigated linear PS samples PS22K and PS39K were used in this study.22 These samples were synthesized as precursors of the 4-arm star PS samples 4S22 and 4S39 by living anionic polymerization. The weight-average molar masses Mw were determined by static light scattering in benzene at 25 °C to be 2.18 × 104 and 3.88 × 104 for PS22K and PS39K, respectively, and the ratio of Mw to the number-average molar mass Mn was determined by size exclusion chromatography to be 1.02 for both samples.22 A cyclic amylose tris(phenylcarbamate) sample, cATPC50K,20 with an Mw of 4.73 × 104 was also used in this study. The three organic solvents, toluene, MEK and THF, were purified by fractional distillation over CaH2.
Small-angle X-ray scattering (SAXS) measurements
SAXS measurements at low temperatures between −80 °C and 40 °C were made at the BL40B2 beamline in SPring-8 (Hyogo, Japan) with a specially designed 2 mmφ capillary cell (Figure 1) with a thermostated nitrogen jet (Cryojet, Oxford Instruments, Abingdon, Oxon, UK). The flow rate of N2 gas was set to be 8000 ml min−1 for the sample flow and 6000 ml min−1 for the shield flow. The distance between the capillary cell and the head of the Cryojet was set to ∼4 mm. The wavelength, camera length and accumulation time were chosen as 0.1 nm, 4000 mm and 120 s, respectively. The temperature of the N2 gas at the capillary was substantially the same as the set temperature above −50 °C, but it was ∼3 °C higher at −80 °C. Thus, we consider the temperature in the cell to be −77 °C when the temperature was set to −80 °C. We were not able to directly determine the temperature in the scattering volume, but the temperature should be substantially close to the N2 gas temperature because the values of the excess scattering intensity divided by the polymer mass concentration ΔI(q)/c at a wide angle were substantially independent of the concentration, although the scattering intensity of the solvent depended to a significant degree on the temperature. The scattering intensities at high temperatures between 15 °C and 70 °C were acquired at the BL-10C beamline at KEK-PF (Ibaraki, Japan) using a 0.15 nm wavelength with the cell holder thermostated by the circulating water bath. A camera length of 2000 mm and an accumulation time of 300 s were chosen for this study. The light scattered from the two X-ray sources was detected using a Rigaku R-AXIS VII imaging plate. The magnitude of the scattering vector q at each pixel on the imaging plate was determined based on the diffraction from silver behenate and/or lead stearate. The scattering intensity I(q) at each q was obtained from the circular average method and was corrected for the incident-beam intensity and the transmittance, both of which were determined using the ionic chambers installed at the upper and lower ends of the cell. The intensity I(q) of the solvent was subtracted from that of the solution in the same capillary to determine the excess scattering intensity ΔI(q). Four solutions with different polymer mass concentrations c were used to extrapolate (c/ΔI(q))1/2 to infinite dilution. The Berry square root plot and the Guinier plot were used to analyze the data for PS and cATPC, respectively, to determine the z-average mean-square radius of gyration <S2>z, the particle scattering function P(q) and the second virial coefficient A2, respectively, because the former plot is suitable to analyze linear flexible chains23, 24 and the latter is suitable for rigid ring polymers.20, 25 Figure 2 shows the Berry plots for PS22K and PS39K in toluene at −77 °C. This figure indicates sufficient accuracy to determine <S2>z and A2, even at low temperature. Note that the optical constant K, including the instrument constant, was determined to obtain A2 from Mw and the reduced intensity (ΔI(0)/c)c=0 at c=0 and q=0 by the relation K[c/ΔI(0)]c=0=Mw−1.
Results and Discussion
Temperature independent chain dimensions of cATPC in THF
Figure 3 displays the Guinier plots [lnP(q) versus q2] for cATPC in THF at 60 °C, 45 °C, 25 °C, −45 °C and −77 °C. The data points at all temperatures investigated here can be fitted by a universal line. The common value of <S2>z1/2 was determined from the initial tangent to be 4.9 nm, which is substantially the same as the literature value (5.1 nm) in 1,4-dioxane at 25 °C.20 Thus, the current cell system may be suitable to investigate the dimensional properties of the polymer in solution over a wide range of temperature. Another point is that the rigid ring amylose derivative cATPC exhibits good solubility in THF, even at −77 °C.
Temperature-dependent chain dimensions of PS in toluene and MEK
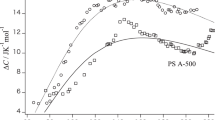
The experimental results of <S2>z in toluene and MEK at various temperatures are presented in Table 1, and the temperature dependences of <S2>z for the two PS samples in toluene and MEK are presented in Figure 4a. Note that the data obtained in toluene at 15 °C are fitted well by the <S2>z–Mw relationship reported by Abe et al.26 (not shown here). Interestingly, the data in MEK do not depend significantly on temperature, with <S2>z exhibiting little change at −40 °C and 70 °C. The Holtzer plots of the MEK data at the two temperatures are almost the same as shown in Figure 5, indicating highly similar chain conformations at the two temperatures.
Temperature dependence of (a) the z-average mean-square radius of gyration <S2>z and (b) the second virial coefficient A2 for PS39K (circles) and PS22K (triangles) in toluene (unfilled symbols) and in 2-butanone (MEK, filled symbols). (c) Temperature dependence of the natural logarithms of the characteristic ratio C∞ for PS in toluene (unfilled circles) and in MEK (filled circles). The broken line has the slope of −1.7 × 10−3 K−1 determined by Osa et al.6 for polystyrene in toluene.
Conversely, the <S2>z are systematically larger in toluene than in MEK. This difference is likely due to the intramolecular excluded-volume effect because the A2 in toluene is significantly larger than that in MEK.27 Indeed, the current A2 data for the two PS samples in toluene are approximately three times larger than those in MEK (see Figure 4b). Note that these A2 values are close to the literature values determined by static light scattering near room temperature.27, 28 While Akcasu and Han29 predicted the Θ temperature in toluene to be −41 °C using an expansion factor of 12 °C, the current A2 data are almost temperature independent, indicating that toluene and MEK are good and intermediate solvents, respectively, even at −77 °C. Thus, we may expect the temperature dependence of the radius expansion factor αs2, which is defined as the ratio <S2>/<S2>0 of the perturbed to unperturbed radii of gyration, to be negligibly small. Therefore, we estimated αs2 in toluene to be 1.28 and 1.40 for PS22K and PS39K, respectively, based on the literature αs2 values in toluene of 15 °C.26 Conversely, αs2 in MEK was estimated to be 1.04 and 1.08 for PS22K and PS39K, respectively. These values were determined from the viscosity expansion factor αη3 in MEK at 35 °C21 because αs2 values are not available for low molar mass PS samples in MEK, and αs2 is mostly the same as αη3 when αη3<1.1 according to the quasi-two-parameter theory,3, 30, 31 with the Domb-Barrett function32 used to determine αs2 and the Barrett function33 used to determine αη3.
The characteristic ratio C∞ can be defined as (<R2>0/nb2)n→∞ or (6<S2>0/nb2)n→∞ where n, b and <R2>0 denote the number of C-C bonds in the main chain, the bond length (0.154 nm) and the unperturbed mean-square end-to-end distance, respectively. Although the two current samples may not have a sufficiently high molar mass to directly obtain C∞, we estimated this value in terms of the wormlike chain model, in which <S2>0 can be expressed as the formula34

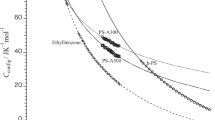
Here, L and λ−1 are the contour length and the stiffness parameter, which is same as the Kuhn segment length (or twice the persistence length) in the conventional wormlike chain model. The former parameter is related to the molar mass M by L=M/ML, with ML representing the molar mass per unit contour length. Assuming the literature ML value of 390 nm−1g mol−1 for PS,35 λ−1 can be estimated using the least-squares method at each temperature, and then C∞ can be estimated by C∞=L/λnb2=M0/2λMLb2, where M0 represents the molar mass of the monomer, leading to the assumption that ML does not result in a significant error of C∞. In actuality, if we assume a 10% larger (or smaller) ML, the resulting C∞ is at most 1% larger (or smaller) than the original value. The helical wormlike chain3 is a better model for PS,36 but the differences between these two models may be negligible for the resulting C∞ determined from the current data because this difference is greater at a lower range of molar mass. Indeed, the 6<S2>0/nb2 values for PS22K and PS39K are close to the obtained C∞, that is, they are only ∼5% and ∼3% smaller than the C∞ values, respectively. Furthermore, P(q) calculated in terms of the touched bead wormlike chain37, 38 model reproduces the experimental data in MEK almost quantitatively, as shown in Figure 5, in which the following parameters were used: ML=390 nm−1g mol−1, λ−1=1.9 nm and dB=1.5 nm. The latter two parameters can be obtained from the curve fitting procedure assuming that ML=390 nm−1g mol−1. The obtained value of λ−1 may be overestimated (∼6%) due to the intramolecular excluded-volume effect, and indeed C∞ is calculated to be 10.7 based on these parameters.
The resulting C∞ data are plotted against temperature in Figure 4c, and the experimental results are summarized in Table 2. These values are fairly close to those in Θ solvents, that is, 10.6 in cyclohexane and 10.1 in trans-decalin calculated from <S2>z versus Mw relationships for high molar mass PS.39, 40, 41, 42 The slightly smaller C∞ values in toluene and MEK might be due to the narrower polydispersity of the current PS samples compared with those in the literature. The data points in toluene monotonically decrease with increasing temperature, and the obtained slope of d lnC∞/dT=−1.0 × 10−3 K−1 is slightly larger than that estimated by Osa et al.6 (dashed line in Figure 4c, d lnC∞/dT=−1.7 × 10−3 K−1) in the same solvent system, and substantially the same as those determined in multiple Θ solvents by Mays et al.5
In the framework of the wormlike chain model, λ−1 can be defined as the ratio of the bending force constant of the wormlike chain to kBT, where kB is the Boltzmann constant. Therefore, d lnC∞/dT is written as

Thus, d lnC∞/dT is calculated to be −3.66 × 10−3 K−1 at 0 °C. This is on the same order as the experimentally determined value. In terms of the helical wormlike chain model, a similar value was obtained at 300 K, that is, d lnC∞/dT=−3.21 × 10−3 K−1.6 In contrast, C∞ in MEK exhibits a minimum ∼10 °C, suggesting that (helical) wormlike or RIS parameters should depend on the temperature, at least in MEK. According to Munk et al.,43 specific interactions between the phenyl ring of PS and the carbonyl group of ethyl acetate reduce the chain dimensions of PS. Furthermore, Mays et al.5 reported that the C∞ in chloroalkanes and diesters are unmistakably smaller than those in cyclohexanes at the same temperature. The positive d lnC∞/dT is thus most likely because the solvation state of PS in MEK depends significantly on temperature in the high temperature range. However, d lnC∞/dT in the lower temperature range becomes similar to that for PS in toluene, most likely because this temperature-dependent solvation behavior is not significant at lower temperatures.
Conclusions
In this study, we successfully obtained the radii of gyration for cATPC and polystyrene (PS) over a wide range of temperature from −77 °C to 70 °C. Whereas the radii of gyration for a rigid ring polymer (cATPC) do not change significantly, those for PS in toluene increase monotonically as the temperature is decreased, and furthermore the temperature coefficient dlnC∞/dT of PS in MEK changes from negative to positive as temperature is increased. This change is most likely due to temperature-dependent polymer–solvent interactions.
References
Flory, P. J. Statistical mechanics of chain molecule. (Interscience (1969).
Kratky, O. & Porod, G. Rontgenuntersuchung geloster fadenmolekule. Recl. Trav. Chim. Pays-Bas. 68, 1106–1122 (1949).
Yamakawa, H. Helicimke chains in polymer solutions, (Springer, 1997).
Yamakawa, H. A new framework of polymer solution science. The helical wormlike chain. Polym. J. 31, 109–119 (1999).
Mays, J. W., Hadjichristidis, N. & Fetters, L. J. Solvent and temperature influences on polystyrene unperturbed dimensions. Macromolecules 18, 2231–2236 (1985).
Osa, M., Kanda, H., Yoshizaki, T. & Yamakawa, H. Temperature coefficients of unperturbed chain dimensions for polystyrene and poly(α-methylstyrene). Polym. J. 39, 423–427 (2007).
Mays, J. W., Hadjichristidis, N., Graessley, W. W. & Fetters, L. J. Temperature dependence of unperturbed dimensions for stereoirregular 1,4-polybutadiene and poly(α-methylstyrene). J. Polym. Sci., Part B: Polym. Phys 24, 2553–2564 (1986).
Mays, J. W., Nan, S. Y., Yunan, W., Li, J. & Hadjichristidis, N. Temperature-dependence of chain dimensions for highly syndiotactic poly(methyl methacrylate). Macromolecules 24, 4469–4471 (1991).
Hong, F., Lu, Y. J., Li, J. F., Shi, W. J., Zhang, G. Z. & Wu, C. Revisiting of dimensional scaling of linear chains in dilute solutions. Polymer. (Guildf). 51, 1413–1417 (2010).
Itou, T., Chikiri, H., Teramoto, A. & Aharoni, S. M. Wormlike chain parameters of poly(hexyl isocyanate) in dilute-solution. Polym. J. 20, 143–151 (1988).
Terao, K., Terao, Y., Teramoto, A., Nakamura, N., Fujiki, M. & Sato, T. Temperature and solvent dependence of stiffness of poly{n-hexyl-[(S)-3-methylpentyl]silylene} in dilute solutions. Macromolecules 34, 4519–4525 (2001).
Teramoto, A., Terao, K., Terao, Y., Nakamura, N., Sato, T. & Fujiki, M. Interplay of the main chain, chiral side chains, and solvent in conformational transitions: Poly{(R)-3,7-dimethyloctyl-(S)-3-methylpentyl silylene}. J. Am. Chem. Soc. 123, 12303–12310 (2001).
Wu, L., Sato, T., Tang, H.-Z. & Fujiki, M. Conformation of a polyfluorene derivative in solution. Macromolecules 37, 6183–6188 (2004).
Yanai, H. & Sato, T. Local conformation of the cellulosic chain in solution. Polym. J. 38, 226–233 (2006).
Izumi, Y., Katano, S., Funahashi, S., Furusaka, M. & Arai, M. Conformation of atactic polystyrene in carbon disulfide observed at a low temperature. Physica B: Condens Matter 180–181 (Part 1), 539–541 (1992).
Izumi, Y., Katano, S., Funahashi, S., Furusaka, M. & Arai, M. Structural study on the sol-gel transition of atactic polystyrene in carbon disulfide. Physica B: Condens Matter 180–181 (Part 1), 545–548 (1992).
Izumi, Y. Structure and mechanism of atactic polystyrene physical gel. Kobunshi Ronbunshu 55, 749–759 (1998).
Tsunashima, Y., Ikuno, M., Onodera, G. & Horii, F. Low-temperature dynamic light scattering. I. Structural reorganization and physical gel formation in cellulose triacetate/methyl acetate dilute solution at -99 - 45°c. Biopolymers 82, 222–233 (2006).
Nakamura, Y., Akashi, K., Norisuye, T., Teramoto, A. & Sato, M. Small-angle x-ray scattering with imaging plate application to dilute polymer solutions. Polym. Bull. 38, 469–476 (1997).
Terao, K., Asano, N., Kitamura, S. & Sato, T. Rigid cyclic polymer in solution: Cycloamylose tris(phenylcarbamate) in 1,4-dioxane and 2-ethoxyethanol. ACS Macro Lett 1, 1291–1294 (2012).
Miyaki, Y. & Fujita, H. Excluded-volume effects in dilute polymer solutions. 11. Tests of the two-parameter theory for radius of gyration and intrinsic viscosity. Macromolecules 14, 742–746 (1981).
Okumoto, M., Terao, K., Nakamura, Y., Norisuye, T. & Teramoto, A. Excluded-volume effects in star polymer solutions: Four-arm star polystyrene in cyclohexane near the theta temperature. Macromolecules 30, 7493–7499 (1997).
Berry, G. C. Thermodynamic and conformational properties of polystyrene.I. Light-scattering studies on dilute solutions of linear polystyrenes. J. Chem. Phys. 44, 4550–4564 (1966).
Terao, K. & Mays, J. W. On-line measurement of molecular weight and radius of gyration of polystyrene in a good solvent and in a theta solvent measured with a two-angle light scattering detector. Eur. Polym. J 40, 1623–1627 (2004).
Terao, K., Shigeuchi, K., Oyamada, K., Kitamura, S. & Sato, T. Solution properties of a cyclic chain having tunable chain stiffness: Cyclic amylose tris(n-butylcarbamate) in Θ and good solvents. Macromolecules 46, 5355–5362 (2013).
Abe, F., Einaga, Y., Yoshizaki, T. & Yamakawa, H. Excluded-volume effects on the mean-square radius of gyration of oligo- and polystyrenes in dilute solutions. Macromolecules 26, 1884–1890 (1993).
Nakata, M. Excluded volume effects in dilute solutions of linear polystyrene. Makromol. Chem. 149, 99–115 (1971).
Einaga, Y., Abe, F. & Yamakawa, H. Second virial coefficients of oligo- and polystyrenes. Effects of chain ends. Macromolecules 26, 6243–6250 (1993).
Akcasu, A. Z. & Han, C. C. Molecular weight and temperature dependence of polymer dimensions in solution. Macromolecules 12, 276–280 (1979).
Yamakawa, H. & Stockmayer, W. H. Statistical mechanics of wormlike chains. II. Excluded volume effects. J. Chem. Phys. 57, 2843–2854 (1972).
Shimada, J. & Yamakawa, H. Statistical-mechanics of helical worm-like chains.25. Excluded-volume effects. J. Chem. Phys. 85, 591–600 (1986).
Domb, C. & Barrett, A. J. Universality approach to expansion factor of a polymer-chain. Polymer. (Guildf). 17, 179–184 (1976).
Barrett, A. J. Intrinsic viscosity and friction coefficients for an excluded volume polymer in the kirkwood approximations. Macromolecules 17, 1566–1572 (1984).
Benoit, H. & Doty, P. Light scattering from non-gaussian chains. J. Phys. Chem. 57, 958–963 (1953).
Norisuye, T. & Fujita, H. Excluded-volume effects in dilute polymer solutions. Xiii. Effects of chain stiffness. Polym. J. 14, 143–147 (1982).
Konishi, T., Yoshizaki, T., Saito, T., Einaga, Y. & Yamakawa, H. Mean-square radius of gyration of oligostyrenes and polystyrenes in dilute-solutions. Macromolecules 23, 290–297 (1990).
Nakamura, Y. & Norisuye, T. Scattering function for wormlike chains with finite thickness. J. Polym. Sci., Part. B: Polym. Phys. 42, 1398–1407 (2004).
Nakamura, Y. & Norisuye, T. In Soft-matter characterization Pecora R., Borsali R (eds. Vol. 1, 236–286 (Springer, 2008).
Miyaki, Y., Einaga, Y. & Fujita, H. Excluded-volume effects in dilute polymer-solutions.7. Very high molecular-weight polystyrene in benzene and cyclohexane. Macromolecules 11, 1180–1186 (1978).
Fukuda, M., Fukutomi, M., Kato, Y. & Hashimot., T. Solution properties of high molecular-weight polystyrene. J. Polym. Sci., Part. B: Polym. Phys. 12, 871–890 (1974).
Inagaki, H., Suzuki, H., Fujii, M. & Matsuo, T. Note on experimental tests of theories for the excluded volume effect in polymer coils. J. Phys. Chem. 70, 1718–1726 (1966).
Fetters, L. J., Hadjichristidis, N., Lindner, J. S. & Mays, J. W. Molecular-weight dependence of hydrodynamic and thermodynamic properties for well-defined linear-polymers in solution. J. Phys. Chem. Ref. Data 23, 619–640 (1994).
Munk, P., Abijaoude, M. T. & Halbrook, M. E. Intrinsic viscosity of polystyrene in mixed solvents. J. Polym. Sci., Polym. Phys. Ed. 16, 105–115 (1978).
Acknowledgements
The synchrotron radiation experiments were performed at the BL40B2 in SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Nos. 2012A1059, 2012B1050 and 2013A1046) and at the BL-10C in KEK-PF under the approval of the Photon Factory Program Advisory Committee (No. 2011G557). We thank Dr Noboru Ohta (SPring-8) for his help in setting up the SAXS apparatus in SPring-8, Ms Xin Yue Jiang (Osaka University) for performing preliminary SAXS measurements, and Professor Takahiro Sato (Osaka University) for fruitful discussion. This work was supported by JSPS KAKENHI Grant Numbers 23750128 and 25410130.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terao, K., Morihana, N. & Ichikawa, H. Solution SAXS measurements over a wide temperature range to determine the unperturbed chain dimensions of polystyrene and a cyclic amylose derivative. Polym J 46, 155–159 (2014). https://doi.org/10.1038/pj.2013.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2013.76
Keywords
This article is cited by
-
Chain stiffness of cellulose tris(phenylcarbamate) in tricresyl phosphate (TCP)
Polymer Bulletin (2018)