Abstract
The fetal zone of the adrenal gland is known to persist after preterm birth, but there is uncertainty as to how long adrenal fetal zone steroid production continues and how it is regulated. The purpose of this study was to test two hypotheses. First, that the urinary excretion of 3β-OH-5-ene steroids persists until term, and then declines, as it does in full-term infants. Second, that the persistence of the fetal zone is due to continuing ACTH stimulation. A longitudinal observational study was undertaken in 22 preterm infants of 24-31-wk gestation. Sequential measurements were made of urinary 3β-OH-5-ene steroids (fetal zone steroid metabolites), plasma dehydroepiandrosterone sulfate (DHEAS), and ACTH. Excretion of urinary 3β-OH-5-ene steroids was 1500-2000 µg kg-1 d-1, persisting until term, and declining abruptly at ∼42 wk postconceptional age (PCA), to levels comparable to term infants at the same PCA. Median plasma ACTH levels rose from <7.6 pg mL-1 at 25-wk PCA to 34.5 pg mL-1 at 46-wk PCA. Urinary 3β-OH-5-ene steroids were highest when ACTH levels were lowest, and were declining when ACTH was rising. In four infants given dexamethasone, urinary excretion of 3β-OH-5-ene steroids and plasma DHEAS were not suppressed fully, when plasma ACTH and cortisol, and urinary cortisol metabolites were. These data suggest that ACTH is not the sole regulator of the adrenal fetal zone steroid synthesis and that involution of the fetal zone is related to gestation rather than birth.
Similar content being viewed by others
Main
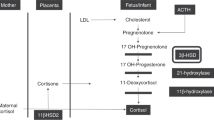
The most striking feature of the adrenal gland of the fetus and newborn infants is its large size. This is due to the presence of the fetal zone of the adrenal cortex, whose mass in utero increases up to term, and then declines rapidly after birth. The main steroid products of the adrenal fetal zone are androgens, such as DHEAS, because of the low activity of 3β-HSD (EC 1.1.1.145) in this zone of the cortex. In utero, fetal DHEAS and 16α-OH DHEAS (the latter hydroxylated in the fetal liver), provide the placenta with the substrates to synthesize estrogens via placental sulfatase and aromatase (1,2). When human fetal adrenal cells are cultured in vitro the activity of 3β-HSD increases with time (3–5), but activity is lowered by the presence of placental tissue (6) and also by estrogens (7,8). This suggests that low 3β-HSD activity is due to enzyme inhibition, although the enzyme concentration is also known to be low in human adrenal fetal zone cells (9). The low activity of 3β-HSD ensures that pregnenolone is diverted to the synthesis of DHEAS maintaining the production of substrate for placental estrogens. In full term infants, plasma DHEAS and urinary excretion of fetal adrenal 3β-OH-5-ene steroids (derived almost exclusively from DHEAS) declines after delivery (10,11), and this is supposed to be due to the release of 3β-HSD from the inhibitory effects of placental estrogen.
Since the observations that decapitation of rabbit and rat fetuses causes adrenal atropy (12,13) and that there is atrophy of the fetal zone in human anencephalic babies (14–16), it has been assumed that ACTH is the main, if not the only, stimulus to the enlargement of the adrenal gland during fetal life. Culture studies also confirm the stimulatory effect of ACTH on steroid production in human fetal adrenal tissue in vitro (5,17). In utero, plasma ACTH concentrations are in the order of 60 pg mL-1 in the second trimester, and rise in the third trimester with increasing gestation (18). These levels are not as high as previously thought (19), but are still close to the upper limit of the normal adult range (20). Although fetal cortisol production is not prevented by the low activity of 3β-HSD, the fetal plasma cortisol levels are low during the second and early part of the third trimester of pregnancy (21), probably due to the high dehydrogenase activity of 11β-hyroxysteroid dehydrogenase type 2, which converts cortisol to cortisone.
In preterm infants the fetal zone is thought not to involute as it does in full-term infants because the plasma levels of DHEAS remain elevated, and there is persistent excretion of its metabolites (3β-OH-5-ene steroids) in the urine (22–26). If ACTH is solely responsible for the persistent production of 3β-OH-5-ene steroids by the fetal zone in preterm infants, it might be expected that the ACTH levels would remain as high after birth as they do in utero, only declining as the fetal zone involutes.
The purpose of this study was to test two hypotheses. First, that the urinary excretion of 3β-OH-5-ene steroids persists until term, and then declines, as it does in full-term infants. Second, that the persistence of the fetal zone is due to continuing ACTH stimulation. To test these hypotheses, sequential measurements of plasma ACTH and DHEAS and of urinary of 3β-OH-5-ene steroids were made in preterm infants up to, and where possible, past, term. It was possible, in some infants, to observe the effects of dexamethasone administration (sufficient to suppress ACTH and cortisol production) on the concentrations of plasma DHEAS and excretion of urinary of 3β-OH-5-ene steroids.
METHODS
Study infants. Twenty-two infants, 12 male and 10 female, were studied for a median of 7 wk (range, 1-27 wk) from birth until discharge from the hospital. Their median birth weight was 895 g (range, 590-1500 g), and their median gestational age, 29 wk (range, 24-31 wk). Eight infants had birth weights below the 10th centile. Nineteen of the infants were ventilated, in six cases this was for 3 d or less. Four infants, had pneumothoraces in the first week, but in only one of these did problems persist beyond the second week of life. Five infants had significant intraventricular hemorrhages in the first week of life, and one of these required treatment for posthemorrhagic hydrocephalus. Seven infants were still in oxygen at a corrected age of 36-wk gestation. Five infants died, three of these at the end of the first week of life, and the other two at 5 and 12 mo age from chronic lung disease and long-term complications of necrotizing enterocolitis, respectively. Only one mother received a single dose of dexamethasone shortly before delivery, as antenatal steroids were not used routinely at the time, and three infants were born to mothers who took prednisolone during pregnancy. Two infants were given dexamethasone postnatally for chronic lung disease, and their results during steroid treatment have been analyzed separately. Two additional infants of 24-wk gestation were studied from 28-31 d of age, for 5-6 wk, to observe the effects of postnatal dexamethasone. The study was approved by the University College London Medical School Ethical committee, and informed consent was obtained from the parents.
Samples. Blood was taken from the umbilical cord, and thereafter from an indwelling arterial line, or peripheral vein, as close to 2 h of age as possible, at 24 h of age, on d 3 and 7, and once a week as close to 0900 h as possible. Samples were collected on ice and centrifuged at 4°C, and the plasma was separated immediately and frozen at -80°C. The samples were later transferred to a freezer held at -25°C. Urine was collected continuously for the first 7 d of life, and 24-h collections were made at weekly intervals thereafter. Urine was continuously aspirated from a purpose-made plastic urine bag, into an Erlenmeyer flask on ice using a diaphragm pump and silicone rubber tubing (27). Samples contaminated with feces were discarded. One infant with severe glycosuria did not have a urinanalysis.
Plasma DHEAS, ACTH, and cortisol assays. Plasma DHEAS was measured using a RIA kit (St. Thomas's Hospital, STRIA, Department of Chemical Pathology, London). The lower limit of detection was 0.6 µmol L-1 (28). The intraassay coefficient of variation was 5% at 3.7 µmol L-1 and 4% at 12.7 µmol L-1. The interassay coefficient of variation was 15% at 3.7 µmol L-1, and 16% at 12.7 µmol L-1. Plasma ACTH was measured by an immunoradiometric assay (29). The lower limit of detection was 7.6 pg mL-1. Intraassay coefficient of variation was 9% at 10 pg mL-1, 3% at 100 pg mL-1, and 2% at 1000 pg mL-1. Plasma cortisol was measured by direct RIA (BioClin Cortisol Radioimmunoassay, Cardiff, UK). The lower limit of detection for this assay was 50 nmol L-1. The intraassay coefficient of variation was 6.2, 4.5, and 4.3% at cortisol concentrations of 44.7, 368, and 574 nmol L-1, respectively. The interassay coefficient of variation was 13.9, 8.4, and 6.6% at the same cortisol concentrations.
Urine steroid analysis. Urine steroid metabolites were analyzed by gas chromatography/mass spectrometry (30), after solid phase extraction with C18 Sep-Pak silica cartridges (Waters Associates, Harrow, Middlesex) and elution with ethanol. Free steroid/glucuronide and sulfate fractions were separated on columns of Sephadex LH-20. A United Technologies Packard gas chromatograph model 437A was used, with a Chrompack (7450) WCOT fused silica column. The lower limit of detection was 500 pg of injected steroid, precision data have been published previously (31). The mass spectrometer was a Hewlett Packard HP 59970 Chemstation (Pascal series).
Statistical methods. Trends in hormone levels with increasing age were examined by multiple regression on both postnatal age and dummy variables identifying each infant. Spearman rank correlation was used to test whether hormone levels at around 1 wk of age were related to gestational age. The Mann Whitney U test was used to determine the significance of perinatal events on the peak values of the parameters measured.
RESULTS
Changes in urinary steroid metabolites after birth. The individual steroids used to calculate total 3β-OH-5-ene steroid excretion are listed in Table 1. The results of urinary steroid analysis plotted as a function of postnatal age are given in Figure 1A. The 3β-OH-5-ene steroids comprised 90% of the total steroid metabolites in the urine, although this percentage declined after 112 d of age. Median 3β-OH-5-ene steroid excretion in the urine increased during the first 3 d of life, probably because of the increase in GFR that occurs after birth. The median then remained fairly constant averaging ∼80 µg kg-1 h-1 (∼2000 µg kg-1 d-1) until 112 d postnatal age when it declined steeply reaching ∼14 µg kg-1 h-1 (∼330 µg kg-1 d-1). Figure 1B shows the data plotted by PCA (strictly speaking postmenstrual age) to demonstrate the influence of maturity. The results from the first 5 d shown in Figure 1A have been excluded to remove the effects of birth, and postnatal changes in renal function. The 3β-OH-5-ene steroids comprised 90% of the total steroid metabolites in the urine until 42-wk PCA, falling thereafter to 55% by 12 wk past term. The median remained elevated averaging ∼80 µg kg-1 h-1 and then declined at about 42-wk gestation. In general, the individual 3β-OH-5-ene steroids which made up the total excretion followed a similar pattern.
(A) Changes in urinary 3β-OH-5-ene steroid excretion of urine steroid metabolites with postnatal age. The solid lines indicate the median. (B) Changes in urinary 3β-OH-5-ene steroid excretion with PCA. The solid lines indicate the median. Measurements made in the first 5 d of life have been excluded.
Plasma. The results of the plasma DHEAS and ACTH measurements are given in Figure 2. After birth the levels of DHEAS increased to a median of ∼10 µmol L-1 and then fell to a median of 3.2 µmol L-1 by 24 h, declining slowly thereafter to undetectable levels in most instances by 140 d. The median plasma ACTH increased steeply after birth, falling below detection limit (<7.6 pg mL-1) on the second day of life, after which it increased slowly to a median of 30 pg mL-1 by 112 d. During this time a substantial number of samples contained no detectable ACTH. Plasma DHEAS and ACTH plotted as a function of PCA are shown in Figure 3, again omitting the results form the first 5 d of life, to remove the changes thought to be due to the stress of birth shown in Figure 2. Plasma DHEAS tended to decline with PCA falling to barely detectable levels at 48 wk PCA, whereas plasma ACTH levels rose. Multiple regression analysis was used to test for trends in plasma DHEAS and ACTH levels with increasing age within infants, and this confirmed a positive trend for ACTH (t = 4.61, p < 0.001) and a negative trend for DHEAS (t = -9.75, p < 0.001). Hormone levels at 6-10 d of age were examined for association between the gestational age at birth and the hormone level immediately after the first week peak. The Spearman rank correlations for these were -0.03 (NS) for ACTH and -0.62 (p < 0.01) for DHEAS.
Effects of postnatal steroid administration. Figure 4 shows an example of changes in plasma ACTH, cortisol, and DHEAS, and urinary 3β-OH-5-ene steroids as a function of postnatal age in one of two study infants who were treated with steroids for chronic lung disease. The measurements made while the infants were treated with steroids have been excluded from the analyses shown in Figures 1–3. The case shown in Figure 4 received sufficient steroids to suppress plasma ACTH and cortisol, but neither the plasma DHEAS nor the urinary 3β-OH-5-ene steroids were suppressed completely. Indeed the urinary 3β-OH-5-ene steroid excretion did not fall below 1000 µg kg-1 d-1 and actually increased during steroid administration. The second study infant of 29-wk gestation, who was small for gestational age, was given two courses of steroids for chronic lung disease. Although there was evidence of suppression of plasma ACTH and cortisol, there was incomplete suppression of plasma DHEAS. Mean urinary 3β-OH-5-ene steroid excretion levels were maintained at 843 µg kg-1 d-1 during the first course of dexamethasone, but fell from 382 to 103 µg kg-1 d-1 during the second course when he has was 10 wk of age (39-wk gestation). Two more infants of 24-wk gestation out with the main study group, were studied just before, and during, postnatal dexamethasone treatment, which started at 4 wk of age. Both of these infants maintained mean urinary 3β-OH-5-ene steroid excretion levels of 776 and 578 µg kg-1 d-1 during the 4 wk of treatment, having had levels of 756 and 663 µg kg-1 d-1, respectively before treatment (Table 2). Again suppression of plasma cortisol and ACTH was confirmed, although plasma cortisol was just detectable on one occasion in infant 4. In all four cases, urinary cortisol/cortisone metabolites were suppressed.
The effect of perinatal events. The Mann Whitney U test was used to determine the significance of certain postnatal events on the peak plasma level of cortisol, ACTH, and DHEAS in the first week, and the total urinary excretion of 3β-OH-5-ene steroids in the first week of life. The infants of mothers who had received antenatal steroids had significantly lower peak ACTH levels than those of mothers who did not (p = 0.042), but there was no difference in total 3β-OH-5-ene steroid excretion, or plasma cortisol. Labor was associated with a significant increase in total 3β-OH-5-ene steroid excretion (p < 0.005). There was no correlation between birth weight and peak plasma DHEAS, or total first week 3β-OH-5-ene steroid excretion using Spearman's rank correlation, nor was there any difference in these parameters in infants with a birth weight <10th centile when compared with those above, using the Mann Whitney U test. Those with intraventricular hemorrhage had significantly lower plasma cortisol and ACTH (p < 0.05, p = 0.005, respectively), but no difference in 3β-OH-5-ene steroid excretion. The peak plasma levels used in the analysis were taken in the first 2 d of life, but head ultrasound scans were not performed within this time, making it impossible to time the onset of intaventricular hemorrhage precisely, or allow speculation as to whether reduced plasma cortisol and ACTH were risk factors for, or effects of, the intraventricular hemorrhage. There was no statistically significantly effect of sex, hyaline membrane disease, pneumothorax, subsequent development of necrotizing enterocolitis, or death, on peak plasma cortisol, ACTH, DHEAS, or total 3β-OH-5-ene steroid excretion in the first week.
DISCUSSION
The conclusions that can be drawn from an observational study of this sort are obviously limited, but in dealing with such an abnormal population no other approach is feasible. The virtually unselected sample of infants studied was 10% of all the surviving infants of <1500 g during the investigation, and it is hoped that they are representative. The only control population is the fetus of equivalent gestation, and because we could not easily study them we have to rely on previously published data.
Persistence of the fetal zone of the adrenal gland after preterm birth. The levels of plasma DHEAS and urinary 3β-OH-5-ene steroids remained elevated for many weeks after birth confirming earlier observations (22–26), but as we were able to follow them past term we observed their spontaneous decline. Because the plasma concentration of DHEAS is determined by both the rate of synthesis and the rate of disposal, when the plasma DHEAS is fluctuating widely (e.g. Fig 4), the urinary excretion of the 3β-OH-5-ene steroids is probably a more accurate measure of the rate of synthesis of DHEAS and therefore activity of the fetal zone.
The median urinary excretion of 3β-OH-5-ene steroids remained elevated until 42-wk gestation and then declined to values comparable to those observed in full-term infants at the same PCA (11). There was a wide variation in 3β-OH-5-ene steroid excretion at each PCA, but individual babies showed similar trends with time, although three infants born at 29-31-wk gestation showed a slight decrease in 3β-OH-5-ene steroid excretion by 35-36-wk PCA. The data are consistent with our first hypothesis and suggest that involution of the fetal adrenal cortex, and by inference the rise in activity of 3β-HSD, is determined by a biologic clock regulated by the duration of gestation rather than by the event of birth, which eliminates the inhibitory action of placental estrogens on 3β-HSD.
The role of ACTH. The second hypothesis to be tested was that the persistent activity of the fetal zone was maintained by ACTH control. From the data given above there is no evidence to support this hypothesis. In the first place levels of plasma ACTH were lower than those reported in the fetus of equivalent PCA (18). After birth both plasma DHEAS and plasma ACTH rose, presumably in response to the stress of delivery. The rise in plasma DHEAS might reflect an acute response to a rise in ACTH, but there was no direct correlation between peak DHEAS and ACTH levels (Spearman's rank correlation), and there was no significant difference between peak DHEAS in infants born with or without labor. This suggests that is is not labor itself that is responsible for a rapid postnatal rise in DHEAS, and the rise could be related to an abrupt loss of placental clearance. Plasma DHEAS remained elevated at a time when ACTH was undetectable (Fig. 2). As Figure 3 shows, there was a tendency for ACTH to rise and DHEAS to fall with increasing PCA. For ACTH, analysis suggests that this is largely due to increasing levels in individual infants as they grow older. However, in the case of DHEAS the explanation appears to involve not just a decrease with age in individuals but a tendency for preterm infants to have higher levels at an early postnatal age. The urinary 3β-OH-5-ene steroids were highest when ACTH levels were lowest and only began to decline at 42-wk PCA, at a time when ACTH was still rising. In Figure 4, for example, the 3β-OH-5-ene steroid excretion was highest in the first week when the ACTH levels were at their lowest.
Doses of exogenous steroids, on a surface area basis more than 10 times those required to suppress the adrenal gland completely in children (32), suppressed the plasma ACTH and cortisol, but failed to demonstrate any unequivocal effect on the plasma DHEAS or urinary excretion of 3β-OH-5-ene steroids. These data are not consistent with the hypothesis that ACTH is the principal regulator of the fetal zone of the adrenal cortex. It cannot be asserted that steroid administration had no effect on the synthesis of 3β-OH-5-ene steroids, and reduction in plasma DHEAS levels in preterm infants on dexamethasone has previously been reported (33), although urinary excretion of the 3β-OH-5-ene steroids is probably a more accurate measure of fetal zone activity. In the case shown in Figure 4 steroid administration may indeed have had some effect on the synthesis of 3β-OH-5-ene steroids, but nothing like the immediate effect on plasma cortisol and ACTH. The effect of dexamethasone appeared to change with maturity in that the four infants treated at a corrected gestation of 28-33 wk maintained 68-87% of their pretreatment urinary 3β-OH-5-ene steroid levels during dexamethasone treatment (Table 2), whereas when infant 2 was treated again at a corrected gestation of 39 wk, mean 3β-OH-5-ene steroid excretion was only 27% of the pretreatment value. It therefore seems possible that the activity of the fetal zone is to some extent independent of ACTH stimulation before term.
These results reveal an apparent dissociation between adrenal androgen and cortisol secretion. Such a dissociation has already been suggested in the perinatal period in rhesus monkeys (34) and in infants exposed to antenatal steroids (35), around the time of adrenarche, and in some adult conditions. At adrenarche the change in circulating concentrations of adrenal androgens, without an apparent change in ACTH or glucocorticoid levels, suggests either a change in adrenal response to ACTH (36), or a cortical androgen-stimulating hormone other than ACTH (37), although no such factor has yet been identified. There is also some evidence that glucocorticoid administration before adrenarche does not suppress serum DHEAS (36,38). Dissociation between adrenal androgen and cortisol secretion has been reported in adults on alternate day prednisone for systemic lupus erythematous, in secondary adrenal failure, in diabetes mellitus, and in Cushing's syndrome (39–42).
The persistence in adrenal fetal zone function until term, even if birth occurs prematurely, suggests that involution of the adrenal fetal zone is related to gestation. This implies a role for the adrenal fetal zone in the duration of pregnancy. The finding that gestation may be prolonged when the fetus has anencephaly (43,44) or congenital adrenal hypoplasia (45), and the suggestion that the estriol:progesterone ratio may rise before labor (46–48) would support this theory. Alternatively, or in addition, the fetal zone of the adrenal gland may be important for fetal development, perhaps in terms of maturation or programming of other organ systems. The interaction between DHEA and other systems appears to be quite diverse (49,50). Of particular interest are the interactions between DHEA and the immune system (51,52), the antiglucocorticoid effects of DHEA (49,53), the negative effects of androgens on fetal lung maturation (54), and the possible role of DHEA at the other extreme of life in aging (55) and cardiovascular disease (56,57). Recently, it has been shown that both plasma DHEAS and its urinary metabolites are raised at adrenarche in children of low birth weight, in comparison with children of higher birth weight (58,59), suggesting that programming of adrenal function may occur in utero. This may be of particular importance because birth size has already been linked with the incidence of hypertension, noninsulin-dependent diabetes mellitus, and ischemic heart disease in later life (60), although the mechanism for this apparent intrauterine programming remains obscure.
CONCLUSIONS
In conclusion: 1) The persistent excretion of 3β-OH-5-ene steroids in the urine of preterm infants after birth is confirmed. 2) The excretion of 3β-OH-5-ene steroids declines abruptly at about 42-wk PCA to levels comparable to term infants. 3) There is no relationship between plasma concentrations of ACTH and DHEAS, and the urinary excretion of 3β-OH-5-ene steroids. 4) Plasma ACTH levels are lower than those reported in the fetus of equivalent PCA, but, like the fetus, ACTH levels increase with PCA. 5) The urinary excretion of 3β-OH-5-ene steroids and plasma DHEAS are not suppressed fully by doses of exogenous steroids sufficient to suppress plasma ACTH and cortisol. 6) These data suggest that ACTH is not the sole regulator of the adrenal fetal zone steroid synthesis, and that the involution of the fetal zone is gestation-related rather than birth-related.
Abbreviations
- 3β-HSD:
-
3β-hydroxysteroid Δ5-steroid dehydrogenase
- DHEAS:
-
dehydroepiandrosterone sulfate
- PCA:
-
postconceptional age
References
Pasqualini JR, Cedard L, Nguyen BL, Alsatt E 1967 Differences in the activity of human term placenta sulphatases for steroid ester sulphates. Biochim Biophys Acta 139: 177–179
Bolte E, Mancuso S, Eriksson G, Wiqvist N, Diczfalusy E 1964 Studies on the aromatisation of neutral steroids in pregnant women. I. Aromatisation of C-19 steroids by placentas perfused in situ. Acta Endocrinol 45: 535–559
Kahri AI, Huhtaniemi I, Salmenpera M 1976 Steroid formation and differentiation of cortical cells in tissue culture of human fetal adrenals in the presence and absence of ACTH. Endocrinology 9: 33–41
Simonian MH, Gill GN 1981 Regulation of the fetal human adrenal cortex: effects of adrenocorticotropin on growth and function of monolayer cultures of fetal and definitive zone cells. Endocrinology 108: 1769–1779
Fujieda K, Faiman C, Reyes FI, Winter JSD 1981 The control of steroidogenesis by human fetal adrenal cells in tissue culture. I. Responses to adrenocorticortropin. J Clin Endocrinol Metab 53: 34–38
Voutilainen R, Kahri AI 1980 Placental origin of the suppression of 3β-hydroxysteroid dehydrogenase in fetal zone cells of human fetal adrenals. J Steroid Biochem 13: 39–43
Byrne GC, Perry YS, Winter JSD 1986 Steroid inhibitory effects upon human adrenal 3β-hydroxysteroid dehydrogenase activity. J Clin Endocrinol Metab 62: 413–418
Hirato K, Yanaihara T, Nakayama T 1982 A study of 5-ene-3β-hydroxysteroid dehydrogenase in human foetal adrenal glands. Acta Endocrinol 99: 122–128
Mason JI, Grizzle WE 1995 Immunohistochemical evaluation of the cellular localisation and ontogeny of 3β-hydroxysteroid dehydrogenase/delta 5-4 isomerase in the human fetal adrenal gland. Endocr Res 21: 69–80
de Peretti E, Forest MG 1978 Pattern of plasma dehydroepiandrosterone sulfate levels in humans from birth to adulthood: evidence for testicular production. J Clin Endocrinol Metab 47: 572–577
Shackleton CHL, Gustafsson J-Å, Mitchell FL 1973 Steroids in newborns and infants. The changing pattern of urinary steroid excretion during infancy. Acta Endocrinol 74: 157–167
Jost A 1948 Influence de la decapitation sur le developpement du tractus genital at de surrenales de l'embryon de lapin. C R Soc Biol 142: 273–275
Jost A, Jacquot R, Cohen A 1962 The pituitary control of the fetal adrenal cortex. In: Currie AR, Symington T, Grant JK (eds) The Human Adrenal Cortex. E. & S. Livingstone, Edinburgh, UK, 569–579.
Bernischke K 1956 Adrenals in anencephaly and hydrocephaly. Obstet Gynecol 8: 412–425
Gray ES, Abramovich DR 1980 Morphologic features of the anencephalic adrenal gland in early pregnancy. Am J Obstet Gynaecol 137: 491–495
Young MC, Laurence KM, Hughes IA 1989 Relationship between fetal adrenal morphology and anterior pituitary function. Horm Res 32: 130–135
Branchaud CT, Goodyer CG, Hall CStG, Arato JS, Silman RE, Giroud CJP 1978 Steroidogenic activity of hACTH and related peptides on the human neocortex and fetal adrenal cortex in organ culture. Steroids 31: 557–572
Economides DL, Nicolaides KH, Perry LA, Chard T 1988 Plasma cortisol and adrenocorticotropin in appropriate and small for gestational age fetuses. Fetal Ther 3: 158–164
Winters AJ, Oliver C, Colston C, MacDonald PC, Porter JC 1974 Plasma ACTH levels in the human fetus and neonate as related to age and parturition. J Clin Endocrinol Metab 39: 269–273
Hodgkinson SC, Allolio B, Landon J, Lowry PJ 1984 Development of a non-extracted "two-site" immunoradiometric assay for corticotropin utilising extreme amino- and carboxy-terminally directed antibodies. Biochem J 218: 703–711
Nahoul K, Daffos F, Forestier F, Chartier M, Scholler R 1988 Plasma corticosteroid patterns in the fetus. J Steroid Biochem 29: 635–640
Reynolds JW 1966 Excretion of 16α-hydroxysteroids by premature infants. J Clin Endocr 26: 1251–1256
Wallace AM, Beesley J, Thomson M, Giles CA, Ross AM, Taylor NF 1987 Adrenal status during the first month of life in mature and premature infants. J Endocrinol 112: 473–480
Reiter EO, Fuldauer VG, Root AW 1977 Secretion of the adrenal androgen, dehydoepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants and in children with endocrinologic abnormalities. J Pediatr 90: 766–770
Grueters A, Korth-Schutz S 1982 Longitudinal study of plasma dehydroepiandrosterone sulfate in preterm and fullterm infants. J Clin Endocrinol Metab 55: 314–320
Forest MG, de Peretti E, Bertrand J 1980 Testicular and adrenal androgens and their binding to plasma proteins in the perinatal period: developmental patterns of plasma testosterone, 4-androstenedione, dehydroepiandrosterone and its sulfate in premature and small for date infants as compared with full-term infants. J Steroid Biochem 12: 25–36
Dauncey MJ, Shaw JCL, Urman J 1977 The absorption and retention of magnesium, zinc, and copper by low birth weight infants fed pasteurized breast milk. Pediatr Res 11: 991–997
Ekins RP 1981 The precision profile: its use in radioimmunoassay assessment and design. Ligand Q 4: 33–44
White A, Smith H, Hoadley M, Dobson SH, Ratcliffe JG 1987 Clinical evaluation of a two-site immunoradiometric assay for adrenocorticotrophin in unextracted human plasma using monoclonal antibodies. Clin Endocrinol 26: 41–51
Honour JW 1997 Steroid profiling. Ann Clin Biochem 34: 32–44
Shackleton CHL, Honour JW 1976 Simultaneous estimation of urinary steroids by semi-automated gas chromatography. Investigation of neo-natal infants and children with abnormal steroid synthesis. Clin Chim Acta 69: 267–283
Hindmarsh PC, Brook CG 1985 Single dose dexamethasone suppression test in children: dose relationship to body size. Clin Endocrinol 23: 67–70
Kari MA, Raivio KO, Stenman UH, Voutilainen R 1996 Serum cortisol, dehydroepiandrosterone sulfate, and steroid-binding globulins in preterm neonates: effect of gestation age and dexamethasone therapy. Pediatr Res 40: 319–324
Seron-Ferre M, Jaffe RB 1981 The fetal adrenal gland. Ann Rev Physiol 43: 141–162
Homoki J, Limmer G, Teller WM 1990 Influence of maternal antenatal treatment with betamethasone on the postnatal adrenal status in preterm infants. Proceedings of the 4th Symposium on the Analysis of Steroids, Pecs, Hungary, 333–337.
Rich BH, Rosenfield RL, Lucky AW, Helke JC, Otto P 1981 Adrenarche: changing adrenal response to adrenocorticotropin. J Clin Endocrinol Metab 52: 1129–1136
Parker LN, Odell WD 1979 Evidence for existence of cortical androgen-stimulating hormone. Am J Physiol 236: E616–E620
Kreitzer PM, Blethen SL, Festa RS, Chasalow FI 1989 Dehydroepiandrosterone sulfate levels are not suppressible by glucocorticoids before adrenarche. J Clin Endocrinol Metab 69: 1309–1311
Averginos PC, Cutler GB Jr, Tsokos GC, Gold PW, Feuillan P, Gallucci WT, Pillemer SR, Loriaux DL, Chrousos GP 1987 Dissociation between cortisol and adrenal androgen secretion in patients receiving alternate day prednisone therapy. J Clin Endocrinol Metab 65: 24–29
Cutler GB Jr, Davis SE, Johnsonbaugh RE, Loriaux DL 1979 Dissociation of cortisol and adrenal androgen secretion in patients with secondary adrenal insufficiency. J Clin Endocrinol Metab 49: 604–609
Cohen HN, Paterson KR, Wallace AM, Beastall GH, Manderson WG, MacCuish AC 1984 Dissociation of adrenarche and gonadarche in diabetes mellitus. Clin Endocrinol 20: 717–724
Cunningham SK, McKenna TJ 1994 Dissociation of adrenal androgen and cortisol secretion in Cushing's syndrome. Clin Endocrinol 41: 795–800
Malpas P 1933 Postmaturity and malformations of the fetus. Br J Obstet Gynaecol 40: 1046–1052
Comerford JB 1965 Pregnancy with anencephaly. Lancet 1: 679–680
Roberts G, Cawdery JE 1970 Congenital adrenal hypoplasia. J Obstet Gynaecol Br Cwlth 77: 654–656
Darne J, McGarrigle HHG, Lachelin GCL 1987 Saliva oestriol, oestradiol, oestrone and progesterone levels in pregnancy: spontaneous labour at term is preceded by a rise in the saliva oestriol:progesterone ratio. Br J Obstet Gynaecol 94: 227–235
Darne J, McGarrigle HHG, Lachelin GL 1987 Increased saliva oestriol to progesterone ratio before idiopathic preterm delivery: a possible predictor for preterm labour? BMJ 294: 270–272
Lewis PR, Galvin PM, Short RV 1987 Salivary oestriol and progesterone concentrations in women during late pregnancy, parturition and the puerperium. J Endocrinol 115: 177–181
Regelson W, Kalimi M 1994 Dehydroepiandrosterone (DHEA)-the multifunctional steroid. II. Effects on the CNS, cell proliferation, metabolic and vascular, clinical and other effects. Mechanism of action? Ann NY Acad Sci 719: 564–575
Ebeling P, Koivisto VA 1994 Physiological importance of dehydroepiandrosterone. Lancet 343: 1479–1481
Rook GAW, Hernandez-Pando R, Lightman SL 1994 Hormones, peripherally activated prohormones and regulation of Th1/Th2 balance. Immunol Today 15: 301–303
Regelson W, Loria R, Kalimi M 1994 Dehydroepiandrosterone (DHEA)-the "mother steroid." I. Immunological action. Ann NY Acad Sci 719: 553–563
May M, Holmes E, Rogers W, Poth M 1990 Protection from glucocorticoid induced thymic involution by dehydroepiandrosterone. Life Sci 46: 1627–1631
Torday JS 1990 Androgens delay human fetal lung maturation. Endocrinology 126: 3240–3244
Regelson W, Loria R, Kalimi M 1988 Hormonal Intervention: "buffer hormones" or "state dependency." The role of dehydroepiandrosterone (DHEA), thyroid hormone, estrogen and pophysectomy in aging. Ann NY Acad Sci 521: 260–271
Barrett-Connor E, Khaw KT, Yen SSC 1986 A prospective study of dehydroepiandrosterone sulfate, mortality and cardiovascular disease. N Engl J Med 315: 1519–1524
Barrett-Connor E, Khaw KT 1987 Absence of an inverse relation of dehydroepiandrosterone sulfate with cardiovascular mortality in postmenopausal women [letter]. N Engl J Med 317: 711
Francois I, de Zegher F 1997 Adrnarche and fetal growth. Pediatr Res 41: 440–442
Clarke PM, Hindmarsh PC, Shiell AW, Law CM, Honour JW, Barker DJP 1996 Size at birth and adrenocortical function in childhood. Clin Endocrinol 45: 721–726
Barker DJP 1994 Mothers, Babies and Disease in Later Life. BMJ Publishing Group, London, pp 41–60, 80–83.
Author information
Authors and Affiliations
Additional information
Supported by the Wellcome Trust. The authors thank Prof. Charles Brook for funding addition technical support.
Rights and permissions
About this article
Cite this article
Midgley, P., Russell, K., Oates, N. et al. Adrenal Function in Preterm Infants: ACTH May Not Be the Sole Regulator of the Fetal Zone. Pediatr Res 44, 887–893 (1998). https://doi.org/10.1203/00006450-199812000-00011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199812000-00011
This article is cited by
-
Potential role of increased oxygenation in altering perinatal adrenal steroidogenesis
Pediatric Research (2015)