Abstract
Many female carriers of Fabry disease are likely to develop severe morbidity and mortality. However, by our own estimation, around 80% of female newborns are missed by our current enzyme-based screening approach. Our team’s aim was to develop an improved cost-effective screening method that is able to detect Fabry disease among female newborns. In Taiwan, based on a database of 916,000 newborns, ~98% of Fabry patients carry mutations out of a pool of only 21 pathogenic mutations. An Agena iPLEX platform was designed to detect these 21 pathogenic mutations using only a single-assay panel. A total of 54,791 female infants were screened and 136 female newborns with the IVS4 + 919G > A mutation and one female newborn with the c.656T > C mutation were identified. Using the current enzyme-based newborn screening approach as baseline, around 83% of female newborns are being missed. Through a family study of the IVS4 female newborns, 30 IVS4 adult family members were found to have left ventricular hypertrophy. Ten patients received endomyocardial biopsy and all were found to have significant globotriaosylceramide (Gb3) accumulation in their cardiomyocytes. All of these individuals now receive enzyme replacement therapy. We have demonstrated that the Agena iPLEX assay is a powerful tool for detecting females with Fabry disease. Furthermore, through this screening, we also have been able to identify many disease-onset adult family members who were originally undiagnosed for Fabry disease. This screening helps them to receive treatment in time before severe and irreversible cardiac damage has occurred.
Similar content being viewed by others
Introduction
Fabry disease (MIM 301500) is an X-linked lysosomal storage disorder that results from a deficiency in α-galactosidase A (α-Gal A) activity [1]. α-Gal A is an enzyme involved in the metabolic breakdown of globotriaosylceramide (Gb-3) and reduced activity of this enzyme results in an accumulation of Gb-3 (and its related glycosphingolipids), mainly in the walls of small blood vessels, nerves, dorsal root ganglia, renal glomerular/tubular epithelial cells, and cardiomyocytes [2]. The clinical features of classically affected patients include acroparesthesia, angiokeratoma, and hypohidrosis during early childhood/adolescence and a progression toward renal insufficiency, cardiomyopathy, and cerebrovascular disease during adulthood [3,4,5,6,7].
Patients with later-onset Fabry disease have higher residual enzyme activity levels than those with classical type Fabry disease. They also lack the classic symptoms of Fabry disease and present with relatively fewer or even single symptoms, including hypertrophic cardiomyopathy, renal failure, or cryptogenic stroke, during the later stages of life [8,9,10,11]. The cardiac later-onset phenotype usually presents only as a cardiac manifestation, such as hypertrophic cardiomyopathy, mitral insufficiency, and/or arrhythmias from the fifth decade to the eighth decade of life [9, 12, 13]. The incidence of Fabry disease has been reported to be between 1 in 40,000 and 1 in 117,000 live births among the general population. However, recently, using newborn screening, our team has found a surprisingly high incidence of Fabry mutation in our male infants (around 1/1600) [14]. Furthermore, several studies carrying out newborn screening for Fabry disease have revealed that the prevalence of later-onset Fabry disease is much higher than that of classical Fabry disease [15,16,17,18,19,20,21]. Moreover, our recent study has demonstrated that severe and irreversible cardiac damages can occur before there are significant cardiac manifestations [22]. This finding indicates that late-onset Fabry disease might be a hidden yet important health issue in some human populations
Based on the incidence of Fabry mutation in our male infants (around 1/1471–1/1600) [14, 22], the estimated incidence of the Fabry mutation in female infants should be between 1/700 and 1/800. However, based on the results of our Fabry newborn screening, the incidence of Fabry mutation in female infants is only 1/3756–1/17,500 [14, 22], which is much lower than the estimated incidence based on the male infant results. We have retrospectively analyzed our symptomatic adult female patients with Fabry disease and found that only around 10% of them have enzyme activity that is lower than 25% of the norm, which is the cutoff point of our current enzyme-based newborn screening [23]. This finding indicates that our enzyme-based screening has a high false-negative rate for heterozygous females. Importantly, several other studies have reported similar high false-negative results when enzymatic assays are used to screen female patients [24, 25].
It is clear that a high percentage of heterozygous females are likely to develop vital organ involvement, including the kidneys, heart, and/or brain [26], under these circumstances, and even females with normal plasma enzyme activity may still present with severe manifestations of the disease affecting various different organs [27, 28]. Therefore, it has become important to develop a more reliable method of detecting females who are heterozygous for Fabry disease as part of our newborn screening program.
Using recent advances in molecular genetic high-throughput mutation detection, we have developed a simple, rapid, and reliable molecular method for the identification of the genetic variations present in a female population that contains individuals who are heterozygous for a Fabry mutation. In our previous study, two molecular high-throughput methods, high-resolution melting analysis (HRM) and Sequenom iPLEX (Agena iPLEX), were investigated as part of a screening study for Fabry disease heterozygous females [23, 29]. Both methods showed very good sensitivity when used to identify Fabry heterozygous female during screening. However, HRM require an experienced researcher to analyze the data and involved a wide range of parameters. In particular, it was found that the melting temperature (T m) needed to be adjusted from time to time. Furthermore, HRM is not able to detect specific mutations directly and a further confirmatory procedure, such as Sanger sequencing, is needed. Therefore, Agena iPLEX was chosen as the approach to screening female newborns for GLA mutations as part of a newborn screening studying. The Agena iPLEX assay is a MassARRAY® genotyping platform that analyzes nucleotide variations by mass spectrometry (MALDI-TOF); it uses a distinguishing allele-specific primer to amplify the extension products [30, 31]. This MassARRAY® assay is a powerful and flexible method that allows the detection of up to thousands of gene variations within hundreds of individuals at the same time [30]. However, conversely, the limitation of Agena iPLEX is that it only can detect known mutations that have been designed into the assay panel. In such circumstances novel mutations will be missed if they have not been designed in the assay panel. However, in Taiwan, 98% of Fabry patients have been found to be affected by a panel of only 21 pathogenic mutations based on about 916,000 newborns screened [29]. Therefore a custom-made mass screening approach to Fabry mutation detection using the Agena iPLEX would seem to be a feasible approach in Taiwan. To reduce the cost of screening, we re-designed the iPLEX PCR primers and extension primers to fit all 21 Taiwanese pathogenic mutations and integrated these into a single assay panel. After accuracy and sensitivity analysis, we have been screening female newborns using this new Agena iPLEX panel since 2014.
Materials and methods
Population and methods
A total of 128,883 infants (male: 66,621; female: 62,262) were screened from August 2014 to December 2016 using a number of mandatory tests during Taiwan’s newborn screening program carried out by the Taipei Institute of Pathology. Around 88.5% of the female’s parents (55,102) were willing to pay for the enzyme-based newborn screening of Fabry disease. The female newborns who had accepted the current enzyme-based newborn screening also received the Agena iPLEX screening as an additional service if we were able to obtain the newborn’s parent’s written informed consent. In total, 54,791 female newborns were enrolled in this study under the above conditions. Positive result by the new test were confirmed by direct sequencing of the α-Gal A gene using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and an ABI Prism 3730 Sequencer. This study was approved by the Medical Ethics Committee of Taipei Veterans General Hospital, Taiwan (IRB No. 2014-12-009B).
DNA mass spectrometry
Twenty-one pairs of Agena iPLEX PCR primers (Table 1) and 21 extension primers (Table 2) were designed for the 21 mutations affecting the α-Gal A gene covered in the new test and this was done using Designer v.3.1 software (Sequenom, Inc., CA, USA) initially. However, to reduce the cost of screening, we re-designed the iPLEX PCR primers and extension primers so that all of the 21 Taiwanese pathogenic mutations could be analyzed simultaneously in only one assay panel. The details of iPLEX PCR primers and extension primers are shown in Tables 1 and 2. Genomic DNA was amplified by PCR reaction using the following temperature cycles: 94 °C for 2 min, [94 °C for 30 s, 56 °C for 30 s, 72 °C for 1 min] × 45 cycles, 72 °C for 1 min, then 4 °C. Subsequently, excess dNTPs were removed from the PCR product by treatment with shrimp alkaline phosphatase, then single-base extension was performed using the following temperature cycles: 94 °C for 30 s, [94 °C for 5 s, (52 °C for 5 s, 80 °C for 5 s) × 5 cycles] × 40 cycles, 72 °C for 3 min, then 4 °C. After desalting, the reaction products were spotted for detection using a mass spectrometer (Sequenom’s Agena iPLEX assay) and the data set was then analyzed using Typer V.4.0 software (Sequenom®) [29].
Family studies based on identification via mutations in the newborn
When the system produced a positive signal for 1 of the 21 mutation in any of the female newborns, the newborn’s family members who might carry this mutation were invited to our hospital to determine whether they carried the mutation. Adult (>30 years old) family members who were confirmed to be carrying the IVS4 + 919G > A (IVS4) mutation then received a careful echocardiographic examination. All participants were examined by two experienced cardiologists who were blind to the GLA mutation results. Echocardiography was performed in accordance with the recommendations of the American Society of Echocardiography using ACUSON equipment (Antares, Siemens AG, Munich, Germany; Sono 7500, Hewlett-Packard Company, Palo Alto, California). Left ventricular mass (LVM) was calculated according to the Devereux cube formula [32]. The LVM was normalized against the subject’s height (m) to power 2.7 power (left ventricular mass index [LVMI] = LVM/height2.7) [33]. Left ventricular hypertrophy (LVH) was defined as a LVMI of >51 g/m2.7 in men and a LVMI of >48 g/m2.7 in women. According to our previous study, many IVS4 adults show significant cardiac fibrosis before the development of LVH or perhaps another significant cardiac manifestation [22]. Gadolinium-enhanced cardiac magnetic resonance imaging (GE-CMR) was performed on the IVS4 adults if they were older than 40 years for females and 30 years for males or when LVH had been detected by heart echogram. The IVS4 patients who met the treatment criteria of the Taiwan Fabry disease treatment guidelines, or had late enhancement in the GE-CMR, received an endomyocardial biopsy and histological investigation. The details of the GE-CMR, endomyocardial biopsy, and histological procedures have been reported in a previous publication [22].
Results
Population screening by DNA mass spectrometry
A total of 54,791 female newborns were enrolled in this study. To our surprise, 137 female newborns were found to have a GLA pathogenic mutation, with 136 female newborns carrying the IVS4 mutation and one female newborn carrying the c.656T > A mutation. The IVS4 mutation is a Chinese hot-spot late-onset cardiac type mutation and the c.656T > A is also a late-onset renal type mutation. In this study, the overall incidence of female infants with Fabry mutations would seem to be as high as 1/400, which is much higher than the previously reported incidence (1/3,756) among female newborns, who were identified using the enzyme-based Fabry newborn screening approach [22].
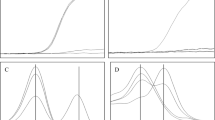
When the results of the enzyme activity assays of these 137 female newborn were retrieved from our enzyme-based Fabry newborn screening system, the mean enzyme activity was found to be 4.03 ± 2.46 μmol/L/h with a range from 1.17 to 15.58 among these female newborns. The distribution of the enzyme activity of these female newborn is presented in Fig. 1. As can be seen, 114 females, made up of 113 females with the IVS4 mutation, and the one female with the c.656T > C, had enzyme activities that were higher than the cutoff point for α-Gal A activity used in our current Fabry newborn screening program. Therefore 83% of the female newborns would seem to have been missed by our current enzyme-based Fabry newborn screening approach.
The incidence of Fabry disease in females as measured by Agena iPLEX assay. There were a total of 54,791 female infants who participated in the mass screening program. In total 136 females with c.639 + 919G > A (IVS4 + 919G > A) and one female with c.656T > C were identified. The incidence of female infants with Fabry mutations is much higher than expected at approximately 1/400. Out of these newborns, 113 females with c.639 + 919G > A would have been missed if screened only using the enzyme-based method, which has a GLA activity cutoff value for female infants of 2.2 μmol/L/hr.
Family study of identified newborns
When we extended our research to include the families of the identified female newborns, the population was made up of the families of 75 female newborns that had been referred to our hospital for a confirmatory diagnosis. On screening members of these families, a total of 113 family members were found to have the IVS4 mutation. Out of these individuals, thirty IVS4 adults had left ventricular hypertrophy. In total, 34 IVS4 adults, consisting of 17 with LVH and 17 without LVH, received GE-CMR and eight of them (6/17 with LVH, 2/17 without LVH) showed delayed enhancement of their hearts. Ten of these received an endomyocardial biopsy and all of them showed significant Gb3 accumulation in their cardiomyocytes. After approval by the committee of the Taiwan Health Insurance Bureau, all of these 10 patients received enzyme replacement therapy and are in a good condition. The details of these 10 patients are presented in Table 3.
Within the family with the c.656T > C mutation, two adult females (the mother and a grandmother) were found to have the same mutation. The grandmother had renal failure and was undergoing hemodialysis. Although the mother had mildly elevated plasma levels of Gb3, there was still no renal involvement at the present moment. It is well known that the c.656T > C is a late-onset renal type mutation and therefore it has been arranged that grandmother to receive enzyme replacement therapy and the mother is undergoing regular follow-ups that assess her renal condition.
Discussion
A large cohort study of Fabry disease has revealed that 77% of women with the disease show neurological involvement, 59% show cardiac involvement, and 40% show renal involvement [32]. However, because of the disease’s atypical clinical manifestations, the diagnosis of female Fabry disease patients is often significantly delayed. Therefore, we believe it is important to detect high-risk females as early as possible and before insidious, but irreversible, damage has affected their vital organs. Furthermore, the present study has indicated that severity of the disease is not completely related to the plasma or leukocyte level of α‐galactosidase A activity in female patients with Fabry disease [32]. This serves to reinforce the point that measurement of α‐galactosidase A activity in plasma or leukocytes has limited diagnostic/prognostic value in females and should not be used to determine treatment options [34].
Therefore the present study, instead of using an enzyme-based screening approach, has developed a simple, rapid, and reliable molecular method for identifying and screening the genetic variations found in female Fabry newborns. The Agena iPLEX assay uses mass spectrometry (MALDI-TOF) analysis is able to provide a rapid and efficient screening system for specific mutations. The approach is able to detect up to several thousand of gene variations in hundreds of individuals at the same time with very high accuracy and sensitivity. Another advantage of the Agena iPLEX assay for newborn screening is that stringent DNA quality control of the samples is not required. Unlike Sanger sequencing or high-resolution melting, where the DNA quality of the samples can severely affect the results, the Agena iPLEX assay has shown great adaptability, even being used with dry blood spot samples. The only disadvantage of the Agena iPLEX assay is that it can only detect known mutations that have been designed into the assay panel. However, In Taiwan, 98% of Fabry patients are known to be affected by only 21 pathogenic mutations after screening 916,000 newborns via newborn screening. In such circumstances a custom-made mass screening for Fabry mutations via the Agena iPLEX system was feasible for Taiwan. Furthermore, as part of this study, rather than using computer-designed primers, we used self-designed PCR and extension primers so that it was possible to analyze all 21 pathogenic mutation in one panel, which reduces the cost of the screening substantially. Therefore, the running costs of this method is only about 10 US dollars per infant during this study. Finally, this study demonstrated that the Agena iPLEX assay is a powerful tool that has high specificity when carrying out rapid screening of known GLA mutations among female newborns.
Our family study showed that out of 75 IVS4 and one c.656T > C families who were referred to our hospital, we were able to identify 115 family member with Fabry mutation and, at the same time, pinpoint 30 IVS4 adults who already had LVH. Ten of these individuals have received cardiac biopsy and started enzyme replacement therapy. Before the development of our new newborn screening system, all of these family members with cardiomyopathy would have never known that they had Fabry disease. These findings highlighted the importance of Fabry female newborn screening. It is useful for a physician to identify undiagnosed Fabry patients who may already have disease-onset, especially the female baby’s fathers with Fabry disease. It is the daughters of these fathers who are frequently missed by current enzyme-based newborn screening approach.
In addition, a recent study has revealed that significant cardiomyocyte Gb3 accumulation in late-onset patients often leads to severe and irreversible cardiac fibrosis before the development of LVH or any other significant cardiac manifestations [22]. It is likely that in many cases it will be too late to start enzyme replacement therapy after the occurrence of LVH or another significant cardiac manifestations when patients have later onset Fabry disease. Therefore, the female newborn screening, including family investigations, means we can identify more individuals carrying GLA mutations and these can then be given appropriate treatment when necessary. Not only are we able to find more undiagnosed patients showing disease-onset, but we also can identified individuals that carrying a pathogenic mutation, but are as yet without disease involvement. With appropriate and careful follow-up so that early disease involvement can be detected, these patients can receive early intervention, which should help to prevent severe and irreversible tissue damage and this will then result in a better treatment outcome.
In this study, we identified 137 IVS4 female newborns. However, our previous studies had demonstrated that most of IVS4 female started to have LVH after they reach the age of 40 years and around one third of these individuals do not seem to develop significant cardiac manifestations of Fabry disease during their whole life [22]. This raises an important ethical issue that needs to be discussed. In spite of the fact that female newborn screening for Fabry disease is likely to help many of the family members who have been misdiagnosed, but show disease-onset of Fabry disease, especially among fathers, do we need to make a diagnosis of Fabry disease among these females at such an early stage in their lives? Furthermore, is there any benefit to these IVS4 females who might never have disease-onset? In addition, another argument is also important, is it justified to use newborn screening to help parents or other relatives to discover they have Fabry disease?
On the other hand, around two-third of females with the most common late-onset mutation suffer significant cardiac manifestations of Fabry disease. Importantly, our previous study revealed that severe cardiac fibrosis may have developed before the occurrence of any cardiac manifestation, when it might be too late to start treatment for Fabry disease. Actually, without this screening, it would be impossible identify these patients early and before they have developed irreversible and significant cardiac manifestations. Furthermore, in an unpublished preliminary study by our group, it has been revealed that the carriers of IVS4 mutation who smoke, have diabetes mellitus, havehyperlipidemia, havehypertension or are obese tend to show an earlier disease onset and to have more severe clinical manifestations. Therefore, it is our hope that our identification of female with the IVS4 mutation at an early stage will allows us to advise them on maintain a healthy life style. This should help to delay disease onset and might even help to avoid disease onset all together. Nevertheless, it is necessary that we have in place a good genetic counseling system that is able to explain the natural course of the disease, together with a good follow-up system and the creation of patient appropriate treatment plans; this should help to appease any unnecessary fears of the parents whose child is undergoing screening.
Since the current screening method based on α-Gal A activity is unable to identify a large percentage of female Fabry patients, an accurate screening method that largely relies on the DNA sequence of the individual being tested is necessary in order to identify female Fabry patients. In this study, the Agena iPLEX assay has been shown to be an effective and reliable method for Fabry newborn screening in the Taiwanese population. This new newborn screening approach has helped us identify many undiagnosed Fabry patients that are showing disease-onset and we have also been able to pinpoint high-risk potential patients who carry a pathogenic mutation, but who as yet show no disease-onset.
References
Desnick RJ, Ioannou YA, Eng CM a-Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York, USA: McGraw-Hill, 2001. p. 3733–74
Clarke JT. Narrative review: Fabry disease. Ann Intern Med. 2007;146:425–33.
Desnick RJ, Wasserstein MP. Fabry disease: clinical features and recent advances in enzyme replacement therapy. Adv Nephrol Necker Hosp. 2001;31:317–39.
Desnick RJ, Brady RO. Fabry disease in childhood. J Pediatr. 2004;144:S20–6.
Zarate YA, Hopkin RJ. Fabry’s disease. Lancet. 2008;372:1427–35.
Weidemann F, Linhart A, Monserrat L, Strotmann J. Cardiac challenges in patients with Fabry disease. Int J Cardiol. 2010;141:3–10.
Bhatia GS, Leahy JF, Connolly DL, Davis RC. Severe left ventricular hypertrophy in Anderson-Fabry disease. Heart. 2004;90:1136.
Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–93.
Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ, Elliott PM. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002;105:1407–11.
Nakao S, Kodama C, Takenaka T, Tanaka A, Yasumoto Y, Yoshida A, et al. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int. 2003;64:801–7.
Rolfs A, Bottcher T, Zshiesche M, Morris P, Winchester B, Bauer P, et al. Prevalence of Fabry disease in patients with cryptogenic stroke: a prospective study. Lancet. 2005;366:1794–6.
Nance CS, Klein CJ, Banikazemi M, Dikman SH, Phelps RG, McArthur JC, et al. Later-onset Fabry disease: an adult variant presenting with the cramp-fasciculation syndrome. Arch Neurol. 2006;63:453–7.
Nagueh SF. Fabry disease. Heart. 2003;89:819–20.
Lin HY, Chong KW, Hsu JH, Yu HC, Shih CC, Huang CH, et al. High incidence of the cardiac variant of Fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet. 2009;2:450–6.
Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–54.
Scott CR, Elliott S, Buroker N, Thomas LI, Keutzer J, Glass M, et al. Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J Pediatr. 2013;163:498–503.
Inoue T, Hattori K, Ihara K, Ishii A, Nakamura K, Hirose S. Newborn screening for Fabry disease in Japan: prevalence and genotypes of Fabry disease in a pilot study. J Hum Genet. 2013;58:548–52.
Wittmann J, Karg E, Turi S, Legnini E, Wittmann G, Giese AK, et al. Newborn screening for lysosomal storage disorders in hungary. JIMD Rep. 2012;6:117–25.
Mechtler TP, Metz TF, Muller HG, Ostermann K, Ratschmann R, De Jesus VR, et al. Short-incubation mass spectrometry assay for lysosomal storage disorders in newborn and high-risk population screening. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;908:9–17.
Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H, et al. High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79:31–40.
Hopkins PV, Campbell C, Klug T, Rogers S, Raburn-Miller J, Kiesling J. Lysosomal storage disorder screening implementation: findings from the first six months of full population pilot testing in Missouri. J Pediatr. 2015;166:172–7.
Hsu TR, Hung SC, Chang FP, Yu WC, Sung SH, Hsu CL, et al. Later-onset fabry disease - cardiac damage progresses in Silence - experience with a highly prevalent mutation. J Am Coll Cardiol. 2016;68:2554–63.
Tai CL, Liu MY, Yu HC, Chiang CC, Chiang H, Suen JH, et al. The use of high resolution melting analysis to detect Fabry mutations in heterozygous females via dry bloodspots. Clin Chim Acta. 2012;413:422–7.
Linthorst GE, Vedder AC, Aerts JM, Hollak CE. Screening for Fabry disease using whole blood spots fails to identify one-third of female carriers. Clin Chim Acta. 2005;353:201–3.
Linthorst GE, Poorthuis BJ, Hollak CE. Enzyme activity for determination of presence of Fabry disease in women results in 40% false-negative results. J Am Coll Cardiol. 2008;51:2082.
Wilcox WR, Oliveira JP, Hopkin RJ, Ortiz A, Banikazemi M, Feldt-Rasmussen U, et al. Females with Fabry disease frequently have major organ involvement: lessons from the fabry registry. Mol Genet Metab. 2008;93:112–28.
Deegan P, Baehner AF, Barba-Romero MA, Hughes DA & Beck M Fabry disease in females: clinical characteristics and the effects of enzyme replacement therapy. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry disease: perspectives from 5 years of FOS. Oxford, UK: Oxford PharmaGenesis, 2006. p. 295–304.
Gupta S, Ries M, Kotsopoulos S, Schiffmann R. The relationship of vascular glycolipid storage to clinical manifestations of Fabry disease: a cross-sectional study of a large cohort of clinically affected heterozygous women. Medicine. 2005;84:261–8.
Lee SH, Li CF, Lin HY, Lin CH, Liu HC, Tsai SF, et al. High-throughput detection of common sequence variations of Fabry disease in Taiwan using DNA mass spectrometry. Mol Genet Metab. 2014;111:507–12.
Calvo SE, Tucker EJ, Compton AG, Kirby DM, Crawford G, et al. High-throughput, pooled sequencing identifies mutations in NUBPL and FOXRED1 in human complex I deficiency. Nat Genet. 2010;42:851–8.
Gabriel S, Ziaugra L, Tabbaa D SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet 2009; Chapter 2: Unit 2.12.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60.
Deegan PB, Baehner AF, Barba-Romero MA, Hughes DA, Kampmann C, Beck M, et al. Natural history of Fabry disease in females in the Fabry outcome survey. J Med Genet. 2006;43:347–52.
Acknowledgements
This work was partially supported by the National Science Council, Taiwan (No. NSC-100-2325-B-010–4) and Taipei Veterans General Hospital (No. V101C-129 and V101C-187).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Lu, YH., Huang, PH., Wang, LY. et al. Improvement in the sensitivity of newborn screening for Fabry disease among females through the use of a high-throughput and cost-effective method, DNA mass spectrometry. J Hum Genet 63, 1–8 (2018). https://doi.org/10.1038/s10038-017-0366-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-017-0366-y
This article is cited by
-
How relevant are cerebral white matter lesions in the D313Y variant of the α-galactosidase A gene? Neurological, cardiological, laboratory, and MRI data of 21 patients within a follow-up of 3 years
Neurological Sciences (2023)
-
Morbus Fabry in der Neurologie
DGNeurologie (2021)
-
Fabry disease screening in high-risk populations in Japan: a nationwide study
Orphanet Journal of Rare Diseases (2020)
-
Prevalence of Fabry disease in dialysis patients: Western Australia Fabry disease screening study - the FoRWARD study
Orphanet Journal of Rare Diseases (2020)