Abstract
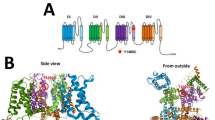
KCNB1 encodes the α-subunit of Kv2.1, the main contributor to neuronal delayed rectifier potassium currents. The subunit consists of six transmembrane α helices (S1–S6), comprising the voltage-sensing domain (S1–S4) and the pore domain (S5-P-S6). Heterozygous KCNB1 pathogenic variants are associated with developmental and epileptic encephalopathy. Here we report an individual who shows the milder phenotype compared to the previously reported cases, including delayed language development, mild intellectual disability, attention deficit hyperactivity disorder, late-onset epilepsy responsive to an antiepileptic drug, elevation of serum creatine kinase, and peripheral axonal neuropathy. On the other hand, his brain MRI showed characteristic findings including periventricular heterotopia, polymicrogyria, and abnormal corpus callosum. Exome sequencing identified a novel de novo KCNB1 variant c.574G>A, p.(Ala192Thr) located in the S1 segment of the voltage-sensing domain. Functional analysis using the whole-cell patch-clamp technique in Neuro2a cells showed that the Ala192Thr mutant reduces both activation and inactivation of the channel at membrane voltages in the range of −50 to −30 mV. Our case could expand the phenotypic spectrum of patients with KCNB1 variants, and suggested that variants located in the S1 segment might be associated with a milder outcome of seizures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Murakoshi H, Trimmer JS. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J Neurosci. 1999;19:1728–35.
Labro AJ, Snyders DJ. Being flexible: the voltage-controllable activation gate of kv channels. Front Pharm. 2012;3:168.
Bishop HI, Guan D, Bocksteins E, Parajuli LK, Murray KD, Cobb MM, et al. Distinct cell- and layer-specific expression patterns and independent regulation of Kv2 channel subtypes in cortical pyramidal neurons. J Neurosci. 2015;35:14922–42.
Torkamani A, Bersell K, Jorge BS, Bjork RL Jr, Friedman JR, Bloss CS, et al. De novo KCNB1 mutations in epileptic encephalopathy. Ann Neurol. 2014;76:529–40.
Kang SK, Vanoye CG, Misra SN, Echevarria DM, Calhoun JD, O'Connor JB, et al. Spectrum of KV 2.1 dysfunction in KCNB1-associated neurodevelopmental disorders. Ann Neurol. 2019;86:899–912.
Bar C, Barcia G, Jennesson M, Le Guyader G, Schneider A, Mignot C, et al. Expanding the genetic and phenotypic relevance of KCNB1 variants in developmental and epileptic encephalopathies: 27 new patients and overview of the literature. Hum Mutat. 2020;41:69–80.
Saitsu H, Akita T, Tohyama J, Goldberg-Stern H, Kobayashi Y, Cohen R, et al. De novo KCNB1 mutations in infantile epilepsy inhibit repetitive neuronal firing. Sci Rep. 2015;5:15199.
Bar C, Kuchenbuch M, Barcia G, Schneider A, Jennesson M, Le Guyader G, et al. Developmental and epilepsy spectrum of KCNB1 encephalopathy with long-term outcome. Epilepsia 2020;61:2461–73.
de Kovel CGF, Syrbe S, Brilstra EH, Verbeek N, Kerr B, Dubbs H, et al. Neurodevelopmental disorders caused by de novo variants in KCNB1 genotypes and phenotypes. JAMA Neurol. 2017;74:1228–36.
Marini C, Romoli M, Parrini E, Costa C, Mei D, Mari F, et al. Clinical features and outcome of 6 new patients carrying de novo KCNB1 gene mutations. Neurol Genet. 2017;3:e206.
Xiong J, Liu Z, Chen S, Kessi M, Chen B, Duan H, et al. Correlation analyses of clinical manifestations and variant effects in KCNB1-related neurodevelopmental disorder. Front Pediatr. 2022;9:755344.
Smith RS, Walsh CA. Ion channel functions in early brain development. Trends Neurosci. 2020;43:103–14.
Barba C, Parrini E, Coras R, Galuppi A, Craiu D, Kluger G, et al. Co-occurring malformations of cortical development and SCN1A gene mutations. Epilepsia 2014;55:1009–19.
Vlachou V, Larsen L, Pavlidou E, Ismayilova N, Mazarakis ND, Scala M, et al. SCN2A mutation in an infant with Ohtahara syndrome and neuroimaging findings: expanding the phenotype of neuronal migration disorders. J Genet. 2019;98:54.
Smith RS, Kenny CJ, Ganesh V, Jang A, Borges-Monroy R, Partlow JN, et al. Sodium channel SCN3A (NaV1.3) regulation of human cerebral cortical folding and oral motor development. Neuron 2018;99:905–13.e7.
Platzer K, Yuan H, Schutz H, Winschel A, Chen W, Hu C, et al. GRIN2B encephalopathy: novel findings on phenotype, variant clustering, functional consequences and treatment aspects. J Med Genet. 2017;54:460–70.
Fry AE, Fawcett KA, Zelnik N, Yuan H, Thompson BAN, Shemer-Meiri L, et al. De novo mutations in GRIN1 cause extensive bilateral polymicrogyria. Brain 2018;141:698–712.
Graber D, Imagawa E, Miyake N, Matsumoto N, Miyatake S, Graber M, et al. Polymicrogyria in a child with KCNMA1-related channelopathy. Brain Dev. 2022;44:173–7.
Miyatake S, Kato M, Kumamoto T, Hirose T, Koshimizu E, Matsui T, et al. De novo ATP1A3 variants cause polymicrogyria. Sci Adv. 2021;7:eabd2368.
Levin M, Pezzulo G, Finkelstein JM. Endogenous bioelectric signaling networks: exploiting voltage gradients for control of growth and form. Annu Rev Biomed Eng. 2017;19:353–87.
Hurni N, Kolodziejczak M, Tomasello U, Badia J, Jacobshagen M, Prados J, et al. Transient cell-intrinsic activity regulates the migration and laminar positioning of cortical projection neurons. Cereb Cortex. 2017;27:3052–63.
Vitali I, Fièvre S, Telley L, Oberst P, Bariselli S, Frangeul L, et al. Progenitor hyperpolarization regulates the sequential generation of neuronal subtypes in the developing neocortex. Cell. 2018;174:1264–76.e15.
Watanabe K, Nakashima M, Kumada S, Mashimo H, Enokizono M, Yamada K, et al. Identification of two novel de novo TUBB variants in cases with brain malformations: case reports and literature review. J Hum Genet. 2021;66:1193–7.
Sano K, Miura S, Fujiwara T, Fujioka R, Yorita A, Noda K, et al. A novel missense mutation of RYR1 in familial idiopathic hyper CK-emia. J Neurol Sci. 2015;356:142–7.
Rubegni A, Malandrini A, Dosi C, Astrea G, Baldacci J, Battisti C, et al. Next-generation sequencing approach to hyperCKemia: a 2-year cohort study. Neurol Genet. 2019;5:e352.
Kyriakides T, Angelini C, Schaefer J, Sacconi S, Siciliano G, Vilchez JJ, et al. EFNS guidelines on the diagnostic approach to pauci- or asymptomatic hyperCKemia. Eur J Neurol. 2010;17:767–73.
Fromer M, Moran JL, Chambert K, Banks E, Bergen SE, Ruderfer DM, et al. Discovery and statistical genotyping of copy-number variation from whole-exome sequencing depth. Am J Hum Genet. 2012;91:597–607.
Nord AS, Lee M, King MC, Walsh T. Accurate and exact CNV identification from targeted high-throughput sequence data. BMC Genom. 2011;12:184.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Lu JM, Zhang JF, Ji CH, Hu J, Wang K. Mild phenotype in a patient with developmental and epileptic encephalopathy carrying a novel de novo KCNB1 variant. Neurol Sci. 2021;42:4325–7.
Speca DJ, Ogata G, Mandikian D, Bishop HI, Wiler SW, Eum K, et al. Deletion of the Kv2.1 delayed rectifier potassium channel leads to neuronal and behavioral hyperexcitability. Genes Brain Behav. 2014;13:394–408.
Shen H, Bocksteins E, Kondrychyn I, Snyders D, Korzh V. Functional antagonism of voltage-gated K+ channel α-subunits in the developing brain ventricular system. Development 2016;143:4249–60.
Yu W, Shin MR, Sesti F. Complexes formed with integrin-α5 and KCNB1 potassium channel wild type or epilepsy-susceptibility variants modulate cellular plasticity via Ras and Akt signaling. FASEB J. 2019;33:14680–9.
Broix L, Jagline H, L Ivanova E, Schmucker S, Drouot N, Clayton-Smith J, et al. Mutations in the HECT domain of NEDD4L lead to AKT-mTOR pathway deregulation and cause periventricular nodular heterotopia. Nat Genet. 2016;48:1349–58.
Wang L, Zhou K, Fu Z, Yu D, Huang H, Zang X, et al. Brain and Akt signaling: the crossroads of signaling pathway and neurodevelopmental diseases. J Mol Neurosci. 2017;61:379–84.
Calhoun JD, Vanoye CG, Kok F, George AL Jr, Kearney JA. Characterization of a KCNB1 variant associated with autism, intellectual disability, and epilepsy. Neurol Genet. 2017;3:e198.
Freites JA, Tobias DJ. Voltage sensing in membranes: from macroscopic currents to molecular motions. J Membr Biol. 2015;248:419–30.
Akita T, Fukuda A. Intracellular Cl− dysregulation causing and caused by pathogenic neuronal activity. Pflug Arch. 2020;472:977–87.
Jackson WF. KV channels and the regulation of vascular smooth muscle tone. Microcirculation. 2018;25. https://doi.org/10.1111/micc.12421.
Vellecco V, Martelli A, Bibli IS, Vallifuoco M, Manzo OL, Panza E, et al. Anomalous Kv 7 channel activity in human malignant hyperthermia syndrome unmasks a key role for H2 S and persulfidation in skeletal muscle. Br J Pharm. 2020;177:810–23.
Shiers S, Klein RM, Price TJ. Quantitative differences in neuronal subpopulations between mouse and human dorsal root ganglia demonstrated with RNAscope in situ hybridization. Pain 2020;161:2410–24.
Hulme AJ, McArthur JR, Maksour S, Miellet S, Ooi L, Adams DJ, et al. Molecular and functional characterization of neurogenin-2 induced human sensory neurons. Front Cell Neurosci. 2020;14:600895.
Zhu X, Chen Y, Xu X, Xu X, Lu Y, Huang X, et al. SP6616 as a Kv2.1 inhibitor efficiently ameliorates peripheral neuropathy in diabetic mice. EBioMedicine 2020;61:103061.
Nardello R, Mangano GD, Miceli F, Fontana A, Piro E, Salpietro V. Age-dependent epileptic encephalopathy associated with an unusual co-occurrence of ZEB2 and SCN1A variants. Epileptic Disord. 2020;22:111–5.
Hiraide T, Ogata T, Watanabe S, Nakashima M, Fukuda T, Saitsu H. Coexistence of a CAV3 mutation and a DMD deletion in a family with complex muscular diseases. Brain Dev. 2019;41:474–9.
Acknowledgements
We would like to thank the patients for participating in this work. This work was supported by Grants-in-Aid for Scientific Research (B) (JP20H03641), (C) (JP20K08236 and JP21K06766), and Grant-in-Aid for Challenging Research (Exploratory) (20K21570) from the Japan Society for the Promotion of Science, and the Takeda Science Foundation.
Author information
Authors and Affiliations
Contributions
TA and HS contributed to the conception and design of the study. TH, TA, KU, SM, MS, MK, and HS contributed to the acquisition and analysis of data. TH, TA, MN, MK, AF, and HS contributed to drafting the text and preparing the figure. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hiraide, T., Akita, T., Uematsu, K. et al. A novel de novo KCNB1 variant altering channel characteristics in a patient with periventricular heterotopia, abnormal corpus callosum, and mild seizure outcome. J Hum Genet 68, 25–31 (2023). https://doi.org/10.1038/s10038-022-01090-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-022-01090-5