Abstract
Background/objectives
Oxylipins are polyunsaturated fatty acid derivatives involved in the regulation of various processes, including chronic inflammation, insulin resistance and hepatic steatosis. They can be synthesized in various tissues, including adipose tissue. There is some evidence that obesity is associated with the deregulation of serum oxylipin levels. The aim of this study was to evaluate the effect of bariatric surgery (one-anastomosis gastric bypass) on the serum levels of selected oxylipins and their fatty acid precursors and to verify the hypothesis that their changes after surgery can contribute to the resolution of inflammation. Moreover, we compared the oxylipin levels (prostaglandin E2, 13-HODE, maresin 1 and resolvin E1), fatty acids and the expression of enzymes that synthesize oxylipins in adipose tissue of lean controls and subjects with severe obesity.
Subjects/methods
The study included 50 patients with severe obesity that underwent bariatric surgery and 41 subjects in lean, control group. Fatty acid content was analyzed by GC-MS, oxylipin concentrations were measured with immunoenzymatic assay kits and real-time PCR analysis was used to assess mRNA levels in adipose tissue.
Results
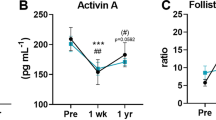
Our results show increased expression of some enzymes that synthesize oxylipins in adipose tissue and alterations in the levels of oxylipins in both adipose tissue and serum of subjects with obesity. After bariatric surgery, the levels of anti-inflammatory oxylipins increased, whereas pro-inflammatory oxylipins decreased.
Conclusions
In patients with obesity, the metabolism of oxylipins is deregulated in adipose tissue, and their concentrations in serum are altered. Bariatric surgery modulates the serum levels of pro- and anti-inflammatory oxylipins, which may contribute to the resolution of inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Boini KM, Xia M, Koka S, Gehr TWB, Li PL. Sphingolipids in obesity and related complications. Front Biosci—Landmark. 2017;22:96–116.
Mika A, Sledzinski T. Alterations of specific lipid groups in serum of obese humans: a review. Obes Rev. 2017;18:247–72.
Al-Sulaiti H, Diboun I, Banu S, Al-Emadi M, Amani P, Harvey TM, et al. Triglyceride profiling in adipose tissues from obese insulin sensitive, insulin resistant and type 2 diabetes mellitus individuals. J Transl Med. 2018;16:1–13.
Wang Y, Huang F. N-3 polyunsaturated fatty acids and inflammation in obesity: local effect and systemic benefit. Biomed Res Int. 2015;2015:581469.
Castagneto-Gissey L, Casella-Mariolo J, Casella G, Mingrone G. Obesity surgery and cancer: what are the unanswered questions? Front Endocrinol (Lausanne). 2020;11:213.
Yamashita AS, Belchior T, Lira FS, Bishop NC, Wessner B, Rosa JC, et al. Regulation of metabolic disease-associated inflammation by nutrient sensors. Mediators Inflamm. 2018;2018:8261432.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7.
Hsieh P-S, Jin J-S, Chiang C-F, Chan P-C, Chen C-H, Shih K-C. COX-2-mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver. Obesity. 2009;17:1150–7.
Martínez-Fernández L, Laiglesia LM, Huerta AE, Martínez JA, Moreno-Aliaga MJ. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015;121:24–41.
Ahima R, Flier J. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–32.
Liakh I, Pakiet A, Sledzinski T, Mika A. Methods of the analysis of oxylipins in biological samples. Molecules. 2020;25:349.
Jacobi D, Stanya K, Lee C-H. Adipose tissue signaling by nuclear receptors in metabolic complications of obesity. Adipocyte. 2012;1:4–12.
Pauls SD, Du Y, Clair L, Winter T, Aukema HM, Taylor CG, et al. Impact of age, menopause, and obesity on oxylipins linked to vascular health. Arterioscler Thromb Vasc Biol. 2021;41:883–97.
Dieckmann S, Maurer S, Fromme T, Colson C, Virtanen KA, Amri EZ, et al. Fatty acid metabolite profiling reveals oxylipins as markers of brown but not brite adipose tissue. Front Endocrinol (Lausanne). 2020;11. https://doi.org/10.3389/fendo.2020.00073.
Schulte F, Asbeutah AA, Benotti PN, Wood GC, Still C, Bistrian BR, et al. The relationship between specialized pro-resolving lipid mediators, morbid obesity and weight loss after bariatric surgery. Sci Rep. 2020;10:1–11.
Liakh Pakiet, Sledzinski Mika. Modern methods of sample preparation for the analysis of oxylipins in biological samples. Molecules. 2019;24:1639.
Melissa Gabbs, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in our understanding of oxylipins derived from dietary PUFAs 1,2. Adv. Nutr. 2015;6:513–40.
Liakh I, Pakiet A, Sledzinski T, Mika A. Methods of the analysis of oxylipins in biological samples. Molecules. 2020;25. https://doi.org/10.3390/molecules25020349.
Pal A, Al-Shaer AE, Guesdon W, Torres MJ, Armstrong M, Quinn K, et al. Eicosapentaenoic acid prevents obesity‐induced metabolic impairments through the host‐genetic dependent effects of resolvin E1. FASEB J. 2020;34:1–1.
Kutzner L, Rund KM, Ostermann AI, Hartung NM, Galano J-M, Balas L, et al. Development of an optimized LC-MS method for the detection of specialized pro-resolving mediators in biological samples. Front Pharmacol. 2019;10. https://doi.org/10.3389/fphar.2019.00169.
González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, et al. Obesity‐induced insulin resistance and hepatic steatosis are alleviated by ω‐3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–57.
Spector AA, Kim H-Y. Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim Biophys Acta—Mol Cell Biol Lipids. 2015;1851:356–65.
Pickens CA, Sordillo LM, Comstock SS, Harris WS, Hortos K, Kovan B, et al. Plasma phospholipids, non-esterified plasma polyunsaturated fatty acids and oxylipids are associated with BMI. Prostaglandins Leukot Essent Fat Acids. 2015;95:31–40.
Keenan AH, Pedersen TL, Fillaus K, Larson MK, Shearer GC, Newman JW. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J Lipid Res. 2012;53:1662–9.
Naoe S, Tsugawa H, Takahashi M, Ikeda K, Arita M. Characterization of lipid profiles after dietary intake of polyunsaturated fatty acids using integrated untargeted and targeted lipidomics. Metabolites. 2019;9:241.
Pickens CA, Sordillo LM, Zhang C, Fenton JI. Obesity is positively associated with arachidonic acid-derived 5- and 11-hydroxyeicosatetraenoic acid (HETE). Metabolism. 2017;70:177–91.
Möller K, Ostermann AI, Rund K, Thoms S, Blume C, Stahl F, et al. Influence of weight reduction on blood levels of C-reactive protein, tumor necrosis factor-α, interleukin-6, and oxylipins in obese subjects. Prostaglandins, Leukot Essent Fat Acids. 2016;106:39–49.
Hernandez-Carretero A, Weber N, La Frano MR, Ying W, Lantero Rodriguez J, Sears DD, et al. Obesity-induced changes in lipid mediators persist after weight loss. Int J Obes. 2018;42:728–36.
Laiglesia LM, Lorente-Cebrián S, Martínez-Fernández L, Sáinz N, Prieto-Hontoria PL, Burrell MA, et al. Maresin 1 mitigates liver steatosis in ob/ob and diet-induced obese mice. Int J Obes. 2018;42:572–9.
Chakrabarti SK, Wen Y, Dobrian AD, Cole BK, Ma Q, Pei H, et al. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. Am J Physiol—Endocrinol Metab. 2011;300. https://doi.org/10.1152/ajpendo.00203.2010.
Clŕria J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol. 2012;189:2597–605.
Mika A, Sledzinski T, Proczko-Stepaniak M, Magkos F. One anastomosis gastric bypass in the treatment of obesity: effects on body weight and the metabolome. In: Faintuch J, Faintuch S, editors. Obesity and diabetes. Springer International Publishing; 2020. p. 777–790.
Pakiet A, Wilczynski M, Rostkowska O, Korczynska J, Jabłonska P, Kaska L, et al. The effect of one anastomosis gastric bypass on branched-chain fatty acid and branched-chain amino acid metabolism in subjects with morbid obesity. Obes Surg. 2020;30:304–12.
Mika A, Wilczynski M, Pakiet A, Kaska L, Proczko-stepaniak M, Stankiewicz M, et al. Short-term effect of one-anastomosis gastric bypass on essential fatty acids in the Serum of obese patients. Nutrients 2020;12. https://doi.org/10.3390/nu12010187.
Halinski LP, Pakiet A, Jablonska P, Kaska L, Proczko-Stepaniak M, Slominska E, et al. One Anastomosis Gastric Bypass Reconstitutes the Appropriate Profile of Serum Amino Acids in Patients with Morbid Obesity. J Clin Med. 2019;9:100.
Mika A, Kaska L, Proczko-Stepaniak M, Chomiczewska A, Swierczynski J, Smolenski RT, et al. Evidence that the length of bile loop determines serum bile acid concentration and glycemic control after bariatric surgery. Obes Surg. 2018;28:3405–14.
Das U. Bioactive lipids in age-related disorders. Adv Exp Med Biol. 2020;1260:33–83.
García-Alonso V, Titos E, Alcaraz-Quiles J, Rius B, Lopategi A, López-Vicario C, et al. Prostaglandin E2 exerts multiple regulatory actions on human obese adipose tissue remodeling, inflammation, adaptive thermogenesis and lipolysis. PLoS ONE. 2016;11:e0153751.
Wolfer AM, Scott AJ, Rueb C, Gaudin M, Darzi A, Nicholson JK, et al. Longitudinal analysis of serum oxylipin profile as a novel descriptor of the inflammatory response to surgery. J Transl Med. 2017;15:83.
Neels JG. A role for 5-lipoxygenase products in obesity-associated inflammation and insulin resistance. Adipocyte. 2013;2:262–5.
Miyauchi E, Tachikawa M, Declčves X, Uchida Y, Bouillot J-L, Poitou C, et al. Quantitative atlas of cytochrome P450, UDP-glucuronosyltransferase, and transporter proteins in jejunum of morbidly obese subjects. Mol Pharm. 2016;13:2631–40.
Chan P-C, Liao M-T, Hsieh P-S. The dualistic effect of COX-2-mediated signaling in obesity and insulin resistance. Int J Mol Sci. 2019;20:3115.
Fried M, Yumuk V, Oppert J-M, Scopinaro N, Torres AJ, Weiner R, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Facts. 2013;6:449–68.
Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509.
Pakiet A, Jakubiak A, Mierzejewska P, Zwara A, Liakh I, Sledzinski T, et al. The effect of a high-fat diet on the fatty acid composition in the hearts of mice. Nutrients. 2020;12:1–20.
Piehler AP, Grimholt RM, Ovstebo R, Berg JP. Gene expression results in lipopolysaccharide-stimulated monocytes depend significantly on the choice of reference genes. BMC Immunol. 2010;11:21.
Tang S, Wan M, Huang W, Stanton RC, Xu Y. Maresins: specialized proresolving lipid mediators and their potential role in inflammatory-related diseases. Mediators Inflamm. 2018;2018. https://doi.org/10.1155/2018/2380319.
Ota T Obesity-induced inflammation and insulin resistance. Front Endocrinol (Lausanne). 2014;5. https://doi.org/10.3389/fendo.2014.00204.
Shearer GC, Walker RE. An overview of the biologic effects of omega-6 oxylipins in humans. Prostaglandins Leukot Essent Fat Acids. 2018;137:26–38.
Aslan M, Aslan I, Özcan F, Erylmaz R, Ensari CO, Bilecik T. A pilot study investigating early postoperative changes of plasma polyunsaturated fatty acids after laparoscopic sleeve gastrectomy. Lipids Health Dis. 2014;13:1–7.
Colson C, Ghandour RA, Dufies O, Rekima S, Loubat A, Munro P, et al. Diet supplementation in ω3 polyunsaturated fatty acid favors an anti-inflammatory basal environment in mouse adipose tissue. Nutrients. 2019;11:1–17.
Culp BR, Titus BG, Lands WEM. Inhibition of prostaglandin biosynthesis by eicosapentaenoic acid. Prostaglandines Med. 1979;3:269–78.
Sima C, Paster B, Van, Dyke TE. Function of pro-resolving lipid mediator resolvin E1 in type 2 diabetes. Crit Rev Immunol. 2018;38:343–65.
Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2276–83.
Martínez-Fernández L, González-Muniesa P, Sáinz N, Escoté X, Martínez JA, Arbones-Mainar JM, et al. Maresin 1 regulates insulin signaling in human adipocytes as well as in adipose tissue and muscle of lean and obese mice. J Physiol Biochem. 2020. https://doi.org/10.1007/s13105-020-00775-9.
Masoodi M, Kuda O, Rossmeisl M, Flachs P, Kopecky J. Lipid signaling in adipose tissue: connecting inflammation & metabolism. Biochim Biophys Acta—Mol Cell Biol Lipids. 2015;1851:503–18.
Pakiet A, Jakubiak A, Czumaj A, Sledzinski T, Mika A. The effect of western diet on mice brain lipid composition. Nutr Metab. 2019;16:81.
Hishikawa D, Valentine WJ, Iizuka-Hishikawa Y, Shindou H, Shimizu T. Metabolism and functions of docosahexaenoic acid-containing membrane glycerophospholipids. FEBS Lett. 2017;591:2730–44.
Irún P, Lanas A, Piazuelo E. Omega-3 polyunsaturated fatty acids and their bioactive metabolites in gastrointestinal malignancies related to unresolved inflammation. a review. Front Pharmacol. 2019;10. https://doi.org/10.3389/FPHAR.2019.00852.
Serhan C, Lu Y, Hong S, Yang R. Mediator lipidomics: search algorithms for eicosanoids, resolvins, and protectins. Methods Enzymol. 2007;432:275–317.
Swierczynski J, Sledzinski T, Slominska E, Smolenski R, Sledzinski Z. Serum phenylalanine concentration as a marker of liver function in obese patients before and after bariatric surgery. Obes Surg. 2009;19:883–9.
Colas R, Sassolas A, Guichardant M, Cugnet-Anceau C, Moret M, Moulin P, et al. LDL from obese patients with the metabolic syndrome show increased lipid peroxidation and activate platelets. Diabetologia. 2011;54:2931–40.
Vangaveti V, Baune BT, Kennedy RL. Hydroxyoctadecadienoic acids: novel regulators of macrophage differentiation and atherogenesis. Ther Adv Endocrinol Metab. 2010;1:51–60.
Ávila-Román J, Arreaza-Gil V, Cortés-Espinar AJ, Soliz-Rueda JR, Mulero M, Muguerza B, et al. Impact of gut microbiota on plasma oxylipins profile under healthy and obesogenic conditions. Clin Nutr. 2021;40:1475–86.
Acknowledgements
This research was funded by the National Science Centre of Poland, grant number NCN 2016/21/D/NZ5/00219; to AM, and by Medical University of Gdansk, grants ST40; to TS.
Author information
Authors and Affiliations
Contributions
A.M. and T.S. conceived and designed the study; M.P.S. and L.K. were responsible for resources and clinical data; I.L., J.K. and A.M. were responsible for methodology; I.L., A.J., A.P., J.K. and A.M. conducted the investigations; A.M. and T.S. interpreted the data; A.P. produced the figures; A.M. produced the tables; A.M., A.P. and T.S. searched the literature and wrote the first draft. All authors contributed to the revision of the first draft, reviewed and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liakh, I., Janczy, A., Pakiet, A. et al. One-anastomosis gastric bypass modulates the serum levels of pro- and anti-inflammatory oxylipins, which may contribute to the resolution of inflammation. Int J Obes 46, 408–416 (2022). https://doi.org/10.1038/s41366-021-01013-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-01013-y