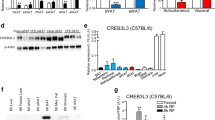
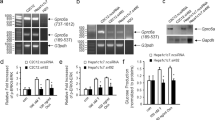
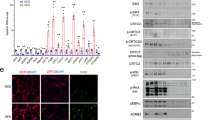
Abstract
Background
The endoplasmic reticulum senses alterations to cellular homeostasis that activates the unfolded protein response (UPR). UPR proteins are known to aid in regulating glucose and lipid metabolism. CREB3 is a UPR-associated transcription factor whose potential role in regulating energy metabolism remains unclear.
Methods
Eight-week-old wild-type (WT) and Creb3+/− mice were placed on control and high-fat diets (HFD) for 8 weeks, and metabolic phenotypes characterized by weekly weighing, indirect calorimetry, body composition scans, glucose tolerance tests, plasma analysis, tissue lipid quantifications and gene/protein expression analysis.
Results
HFD weight gain in Creb3+/− males was reduced by 34% (p < 0.0001) and females by 39.5% (p = 0.014) from their WT counterparts. No differences were found in HFD food intake or total fecal lipids between genotypes. Creb3+/− mice had increased energy expenditure and respiratory exchange ratios (p = 0.002) relative to WT. Creb3+/− mice had significant reductions in absolute fat and lean tissue, while Creb3+/− females had significant reductions in body fat% and increased lean% composition (p < 0.0001) compared to WT females. Creb3+/− mice were protected from HFD-induced basal hyperglycemia (males p < 0.0001; females p = 0.0181). Creb3+/− males resisted HFD-induced hepatic lipid accumulation (p = 0.025) and glucose intolerance compared to WT (p < 0.0001) while Creb3+/− females were protected from lipid accumulation in skeletal muscle (p = 0.001). Despite the metabolic differences of Creb3+/− mice on HFD, lipid plasma profiles did not significantly differ from WT. Fasted Creb3+/− mice additionally revealed upregulation of hepatic energy expenditure and gluconeogenic genes such as Pgc-1a and Gr (glucocorticoid receptor) (p < 0.05), respectively.
Conclusions
Reduced expression of CREB3 increased energy expenditure and the respiratory exchange ratio, and protected mice from HFD-induced weight gain, basal hyperglycemia, and sex-specific tissue lipid accumulation. We postulate that CREB3 is a novel key regulator of diet-induced obesity and energy metabolism that warrants further investigation as a potential therapeutic target in metabolic disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author, RL, upon reasonable request.
References
Basseri S, Austin RC. Endoplasmic reticulum stress and lipid metabolism: mechanisms and therapeutic potential. Biochem Res Int. 2012;2012:841362.
Sampieri L, Di Giusto P, Alvarez C. CREB3 transcription factors: ER-Golgi stress transducers as hubs for cellular homeostasis. Front Cell Dev Biol. 2019;7:123.
Piperi C, Adamopoulos C, Papavassiliou AG. XBP1: a pivotal transcriptional regulator of glucose and lipid metabolism. Trends Endocrinol Metab. 2016;27:119–22.
Zhang C, Wang G, Zheng Z, Maddipati KR, Zhang X, Dyson G, et al. ER-tethered transcription factor CREBH regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress. Hepatology. 2012;55:1070–82.
Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi, et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;18:6755–67.
Chen X, Zhang F, Gong Q, Cui A, Zhuo S, Hu Z, et al. Hepatic ATF6 increases fatty acid oxidation to attenuate hepatic steatosis in mice through peroxisome proliferator–activated receptor α. Diabetes. 2016;65:1904–15.
Usui M, Yamaguchi S, Tanji Y, Tominaga R, Ishigaki Y, Fukumoto M, et al. Atf6α-null mice are glucose intolerant due to pancreatic β-cell failure on a high-fat diet but partially resistant to diet-induced insulin resistance. Metabolism. 2012;61:1118–28.
Lowe CE, Dennis RJ, Obi U, O’Rahilly S, Rochford JJ. Investigating the involvement of the ATF6α pathway of the unfolded protein response in adipogenesis. Int J Obes. 2012;36:1248–51.
Lu R, Yang P, O’Hare P, Misra V. Luman, a new member of the CREB/ATF family, binds to herpes simplex virus VP16-associated host cellular factor. Mol Cell Biol. 1997;9:5117–26.
Raggo C, Rapin N, Stirling J, Gobeil P, Smith-Windsor E, O’Hare P, et al. Luman, the cellular counterpart of herpes simplex virus VP16, is processed by regulated intramembrane proteolysis. Mol Cell Biol. 2002;16:5639–49.
Liang G, Audas TE, Li Y, Cockram GP, Dean JD, Martyn, et al. Luman/CREB3 induces transcription of the endoplasmic reticulum (ER) stress response protein Herp through an ER stress response element. Mol Cell Biol. 2006;21:7999–8010.
DenBoer LM, Hardy-Smith PW, Hogan MR, Cockram GP, Audas TE, Lu R. Luman is capable of binding and activating transcription from the unfolded protein response element. Biochem Biophys Res Commun. 2005;331:113–9.
Audas TE, Li Y, Liang G, Lu R. A novel protein, Luman/CREB3 recruitment factor, inhibits Luman activation of the unfolded protein response. Mol Cell Biol. 2008;12:3952–66.
Minster RL, Hawley NL, Su CT, Sun G, Kershaw EE, Cheng H, et al. A thrifty variant in CREBRF strongly influences body mass index in Samoans. Nat Genet. 2016;48:1049–54.
Chan C, Kok K, Jin D. CREB3 subfamily transcription factors are not created equal: recent insights from global analyses and animal models. Cell Biosci. 2011;1:6.
Ying Z, Zhang R, Verge VM, Misra V. Cloning and characterization of rat Luman/CREB3, a transcription factor highly expressed in nervous system tissue. J Mol Neurosci. 2015;55:347–54.
Satoh A, Han S, Araki M, Nakagawa Y, Ohno H, Mizunoe Y, et al. CREBH improves diet-induced obesity, insulin resistance, and metabolic disturbances by FGF21-dependent and FGF21-independent mechanisms. iScience. 2020;23:100930.
Nakagawa Y, Satoh A, Tezuka H, Han S, Takei K, Iwasaki H, et al. CREB3L3 controls fatty acid oxidation and ketogenesis in synergy with PPARα. Sci Rep. 2016;6:39182.
Nakagawa Y, Shimano H. CREBH regulates systemic glucose and lipid metabolism. Int J Mol Sci. 2018;19:1396.
Nakagawa Y, Satoh A, Yabe S, Furusawa M, Tokushige N, Tezuka H, et al. Hepatic CREB3L3 controls whole-body energy homeostasis and improves obesity and diabetes. Endocrinology. 2014;155:4706–19.
Kim TH, Jo SH, Choi H, Park JM, Kim MY, Nojima H, et al. Identification of Creb3l4 as an essential negative regulator of adipogenesis. Cell Death Dis. 2014;5:e1527.
Penney J, Mendell A, Zeng M, Tran K, Lymer J, Turner, et al. LUMAN/CREB3 is a key regulator of glucocorticoid-mediated stress responses. Mol Cell Endocrinol. 2017;439:95–104.
Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 2016;44:W147–53.
Sanyal AJ, Pacana T. A lipidomic readout of disease progression in a diet-induced mouse model of nonalcoholic fatty liver disease. Trans Am Clin Climatol Assoc. 2015;126:271–88.
Miller KK, Parulekar MS, Schoenfeld E, Anderson E, Hubbard J, Klibanski A, et al. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: the effects of body composition and nutritional intake. J Clin Endocrinol Metab. 1998;83:2309–12.
Baldwin KM, Joanisse DR, Haddad F, Goldsmith RL, Gallagher D, Pavlovich KH, et al. Effects of weight loss and leptin on skeletal muscle in human subjects. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1259–66.
Sha H, He Y, Chen H, Wang C, Zenno A, Shi H, et al. The IRE1α-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009;9:556–4.
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91.
Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–40.
Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem. 2004;136:343–50.
Kim H, Mendez R, Zheng Z, Chang L, Cai J, Zhang R, et al. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor α to regulate metabolic hormone FGF21. Endocrinology. 2014;155:769–82.
Ruan HB, Han X, Li MD, Singh JP, Qian K, Azarhoush S. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1α stability. Cell Metab. 2012;16:226–37.
Acknowledgements
Special thanks to Tiegh Taylor and Dr. Jenna Penney for assistance with experimental procedures. Thank you to the Central Animal Facility at the University of Guelph for mouse colony care and maintenance. Thanks to Dr. Adronie Verbrugghe of the Ontario Veterinary College at the University of Guelph for use of their DXA machine. Thank you to the Analytical Facility for Bioactive Molecules at SickKids Hospital for the TOF-MS Lipids Analysis.
Funding
Funded by Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA), NSERC Collaborative Research & Development and MITACS.
Author information
Authors and Affiliations
Contributions
BSS devised the study, carried out experiments, analyzed data, and wrote the paper. KHDD, AH, and SG performed experiments and analyzed data. RED, MB, and RL contributed to experimental design and paper writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Smith, B.S., Diaguarachchige De Silva, K.H., Hashemi, A. et al. Transcription factor CREB3 is a potent regulator of high-fat diet-induced obesity and energy metabolism. Int J Obes 46, 1446–1455 (2022). https://doi.org/10.1038/s41366-022-01128-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01128-w