Abstract
Objective
To evaluate the efficacy and safety of tin mesoporphyrin (SnMP) in neonates with hyperbilirubinemia (HB) due to hemolysis.
Study Design
This multicenter, placebo-controlled phase 2b study (NCT01887327) randomized newborns (35–42 weeks) with hemolysis started on phototherapy (PT) to placebo (Ctrl), SnMP 3.0 mg/kg, or SnMP 4.5 mg/kg given once IM within 30 min of initiation of PT.
Results
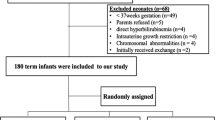
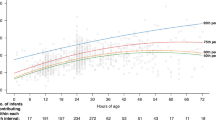
In all, 91 patients were randomized (Ctrl: n = 30; 3 mg/kg SnMP: n = 30; 4.5 mg/kg SnMP: n = 31). At 48 h TSB significantly increased in Ctrl by 17.5% (95% CI 5.6–30.7; p = 0.004) and significantly decreased by −13% (95% CI −21.7 to −3.2; p = 0.013) in the 3.0 mg/kg and by −10.5% (95% CI −19.4 to −0.6; p = 0.041) in the 4.5 mg/kg group. Decreases in SnMP groups were significant (p < 0.0001) vs Ctrl.
Conclusion
SnMP with PT significantly reduced TSB by 48 h. SnMP may be useful as a treatment for HB in neonates with hemolysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kuzniewicz MW, Wickremasinghe AC, Wu YW, McCulloch CE, Walsh EM, Wi S, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504–9.
Vanburg PK, Hansen BM, Greisen G, Mathiasen R, Kasper F, Ebbesen F, et al. Follow-up of extreme neonatal hyperbilirubinaemia in 5- to 10-year-old children: a Danish population-based study. Dev Med Child Neurol. 2015;57:378–84.
Amin SB, Saluja A, Saili A, Orlando M, Wang H, Laroia N, et al. Chronic auditory toxicity in late preterm and term infants with significant hyperbilirubinemia. Pediatrics. 2017;140:e20164009.
Rose J, Vassar R. Movement disorders due to bilirubin toxicity. Semin Fetal Neonatal Med. 2015;20:20–5.
Olds C, Oghalai JS. Audiologic impairment associated with bilirubin-induced neurologic damage. Semin Fetal Neonatal Med. 2015;20:42–6.
LePichon JB, Riordan SM, Watchko J, Shapiro SM. The neurologic sequelae of neonatal hyperbilirubinemia: definitions, diagnosis and treatment of kernicterus spectrum disorders. Curr Pediatr Rev. 2017;13:199–209.
American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316.
Maisels JM, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics 2009;124:1193.
Burgos AE, Schmidt SK, Stevenson DK, Phibbs CS. Readmission for neonatal jaundice in California, 1991–2000: trends and implications. Pediatrics. 2008;121:e864.
Escobar GJ, Greene JD, Hulac P, Kincannon F, Bischoff K, Gardner MN, et al. Rehospitalization after birth hospitalization; patterns among infants of all gestations. Arch Dis Child. 2005;90:125–31.
Bhutani VK. and The Committee on Fetus and newborn. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2011;128:1046–52.
Wolf MF, Childers J, Gray KD, Chivily C, Glenn M, Jones L. Exchange transfusion safety and outcomes in neonatal hyperbilirubinemia. J Perinatol. 2020;40:1506–12.
Zweirs C, Scheffer-Rath ME, Lopriore E, de Haas M, Lilely HG. Immunoglobulin for alloimmune hemolytic disease in neonates. Cochrane Database Syst Rev. 2018;3:CD003313.
Kappas A, Drummond GS, Mamola T, Petmezaki S, Valaes T. Sn-Protoporphyrin use in the management of hyperbilirubinemia in term newborns with direct Coombs-positive ABO Incompatibility. Pediatr. 1988;81:845.
Valaes T, Petmezaki S, Henschke C, Drummond GS, Kappas A. Control of jaundice in preterm newborns by an inhibitor of bilirubin production: studies with Tin-Mesoporphyrin. Pediatrics. 1994;94:1–11.
Kappas A, Drummond GS, Henschke C, Valaes T. Direct comparison of Sn-Mesoporphyrin, an inhibitor of bilirubin production, and phototherapy in controlling hyperbilirubinemia in term and near-term newborns. Pediatrics. 1995;95:468–74.
Valaes T, Drummond GS, Kappas A. Control of hyperbilirubinemia in glucose-6- phosphate dehydrogenase-deficient newborns using an inhibitor of bilirubin production, Sn-mesoporphyrin. Pediatrics. 1998;101:E1.
Kappas A, Drummond GS, Valaes T. A single dose of Sn-mesoporphyrin prevents development of severe hyperbilirubinemia in glucose – 6 – phosphate dehydrogenase-deficient newborns. Pediatrics. 2001;108:25–30.
Bhutani VK, Poland R, Meloy LD, Hegyi T, Fanaroff AA, Maisels MJ. Clinical trial of tin mesoporphyrin to prevent neonatal hyperbilirubinemia. J Perinatol. 2016;36:533–9.
Martinez JC, Garcia HO, Otheguy LE, Drummond CS, Kappas A. Control of severe hyperbilirubinemia in full term newborns with the inhibitor of bilirubin production. Pediatrics. 1999;103:1–5.
Fort FL, Gold J. Phototoxicity of tin protoporphyrin, tin mesoporphyrin, and tin diiododeuteroporphyrin under neonatal phototherapy conditions. Pediatrics. 1989;84:1031–7.
Burke BL, Robbins JM, Mac Bird T, Hobbs CA, Nesmith C, Tilford JM. Trends in hospitalization for neonatal jaundice and kernicterus in the United States, 1988-2005. Pediatrics. 2009;123:524–32.
Fein EH, Friedlander S, Yu Y, Pak Y, Sakai-Bizmark R, Smith LM. Phototherapy for neonatal unconjugated hyperbilirubinemia: Examining outcomes by level of care. Hosp Pediatr. 2019;9:115–20.
Newman TB, Wickremasinghe AC, Walsh EM, Grimes BA, McCulloch CE, Kuzniewicz MW. Retrospective cohort study of phototherapy and childhood cancer in Northern California. Pediatrics. 2016;137:e20151354.
Ramy N, Ghany E, Alsherany W, Nada A, Darwish RK, Rabie WA, et al. Jaundice, phototherapy and DNA damage in full-term neonates. J Perinatal. 2016;36:132–6.
Mokhtar WA, Sherief LM, Elsayed H, Shehab MM, El Gebaly SM, Khalil AMM, et al. Conventional intensive versus LED intensive phototherapy oxidative stress burden in neonatal hyperbilirubinemia of haemolytic origin. Paediatr Child Health. 2020;40:30–4.
Sirota L, Straussberg R, Gurary N, Aloni D, Bessler H. Phototherapy for neonatal hyperbilirubinemia affects cytokine production by peripheral blood mononuclear cells. Eruv J Pediatr. 1999;158:910–3.
Aspberg S, Dahlquist G, Kahan T, Kallen B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21:e733–739.
Dahlquist G, Kallen B. Indications that phototherapy is a risk factor for insulin-dependent diabetes. Diabetes Care. 2003;26:247–8.
Lozado LE, Nylund CM, Gorman GH, Hisle-Gorman E, Erdie-Lalena CR, Kuehn D. Association of autism spectrum disorder with neonatal hyperbilirubinemia. Global Pediatr Health. 2015;2:1–5.
Newman TB, Wu YW, Kuzniewicz MW, Grimes BA, McCulloch CE. Childhood seizures after phototherapy. Pediatrics. 2018;142:e20180648.
Maimburg RD, Olsen J, Sun Y. Neonatal hyperbilirubinemia and the risk of febrile seizures and childhood epilepsy. Epilepsy Res. 2016;124:67–72.
Tyson JE, Pedroza C, Langer J, Green C, Morris B, Stevenson D. Does aggressive phototherapy increase mortality while decreasing profound impairment among the smallest and sickest newborn? J Perinatol. 2012;32:677–84.
Smits-Wintiens VE, Rath ME, van Zwet EW, Oepkes D, Brand A, Walther FJ. Neonatal morbidity after exchange transfusion for red cell alloimmune hemolytic disease. Neonatology. 2013;103:141–7.
Murray NA, Roberts I. Haemolytic disease of the newborn. Arch Dis Child Fetal Neonatal Ed. 2007;92:83–8.
Elsaie AL, Taleb M, Nicosia A, Zangaladze A, Pease ME, Newton K, et al. Comparison of end-tidal carbon monoxide measurements with direct antiglobulin tests in the management of neonatal hyperbilirubinemia. J Perinatol. 2020;40:1513–7.
Acknowledgements
Infacare Pharmaceuticals designed this study to meet Phase 2B requirements of the FDA. They chose clinical sites, provided study oversight, and collected data. They were not involved in the manuscript preparation but have reviewed the manuscript. Data for this article were collected by Infacare but were analyzed independently by the authors.
Author information
Authors and Affiliations
Consortia
Contributions
Dr. Rosenfeld was involved in the study design, drafted the original manuscript and reviewed, and revised the manuscript. Dr. Hudak coordinated and participated in Data analyses, reviewed and revised the manuscript. Dr. Gautam provided statistical analysis and reviewed the manuscript. Dr. Ruiz was involved in study design and reviewed and revised manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol, amendments, and informed consent forms were reviewed and approved by the Institutional Review Boards at each study site. This study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rosenfeld, W.N., Hudak, M.L., Ruiz, N. et al. Stannsoporfin with phototherapy to treat hyperbilirubinemia in newborn hemolytic disease. J Perinatol 42, 110–115 (2022). https://doi.org/10.1038/s41372-021-01223-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01223-2
This article is cited by
-
Is it time for a precision health approach to the management of newborn hyperbilirubinemia?
Journal of Perinatology (2024)