Abstract
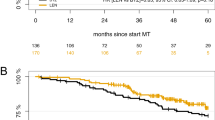
The role of salvage high-dose chemotherapy and autologous stem cell transplantation (sHDCT/ASCT) for relapsed and/or refractory multiple myeloma (RRMM) in the era of continuous novel agent treatment has not been defined. This randomized, open-label, phase III, multicenter trial randomized patients with 1st–3rd relapse of multiple myeloma (MM) to a transplant arm (n = 139) consisting of 3 Rd (lenalidomide 25 mg, day 1–21; dexamethasone 40 mg, day 1, 8, 15, and 22; 4-week cycles) reinduction cycles, sHDCT (melphalan 200 mg/m2), ASCT, and lenalidomide maintenance (10 mg/day) or to a control arm (n = 138) of continuous Rd. Median PFS was 20.7 months in the transplant and 18.8 months in the control arm (HR 0.87; 95% CI 0.65–1.16; p = 0.34). Median OS was not reached in the transplant and 62.7 months in the control arm (HR 0.81; 95% CI 0.52–1.28; p = 0.37). Forty-one patients (29%) did not receive the assigned sHDCT/ASCT mainly due to early disease progression, adverse events, and withdrawal of consent. Multivariate landmark analyses from the time of sHDCT showed superior PFS and OS (p = 0.0087/0.0057) in patients who received sHDCT/ASCT. Incorporation of sHDCT/ASCT into relapse treatment with Rd was feasible in 71% of patients and did not significantly prolong PFS and OS on ITT analysis while patients who received sHDCT/ASCT may have benefitted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gay F, Engelhardt M, Terpos E, Wäsch R, Giaccone L, Auner HW, et al. From transplant to novel cellular therapies in multiple myeloma: EMN guidelines and future perspectives. Haematologica. 2017;103:197-211.
Kumar SK, Callander NS, Alsina M, Atanackovic D, Biermann JS, Chandler JC, et al. Multiple myeloma, version 3.2017, NCCN Clinical Practice Guidelines in oncology. J Natl Compr Canc Netw. 2017;15:230–69.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335:91–7.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Cavo M, Palumbo Antonio, Zweegman Sonja, Dimopoulos Meletios A, Hajek Roman, Pantani Lucia, et al. Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): a randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial). J Clin Oncol. 2016;34:8000-8000.
Atanackovic D, Schilling G. Second autologous transplant as salvage therapy in multiple myeloma. Br J Haematol. 2013;163:565–72.
Cook G, Williams C, Brown JM, Cairns DA, Cavenagh J, Snowden JA, et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [Intensive trial]): a randomised, open-label. Lancet Oncol. 2014;15:874–85.
Cook G, Ashcroft AJ, Cairns DA, Williams CD, Brown JM, Cavenagh JD, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol. 2016;3:e340–51.
Baertsch M-A, Schlenzka J, Mai EK, Merz M, Hillengaß J, Raab MS, et al. Rationale and design of the German-Speaking Myeloma Multicenter Group (GMMG) trial ReLApsE: a randomized, open, multicenter phase III trial of lenalidomide/dexamethasone versus lenalidomide/dexamethasone plus subsequent autologous stem cell transplantatio. BMC Cancer. 2016;16:290.
Durie BGM, Harousseau J-L, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Baertsch M-A, Schlenzka J, Lisenko K, Krzykalla J, Becker N, Weisel K, et al. Cyclophosphamide-based stem cell mobilization in relapsed multiple myeloma patients: a subgroup analysis from the phase III trial ReLApsE. Eur J Haematol. 2017;99. https://doi.org/10.1111/ejh.12888.
Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–23.
Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N. Engl J Med. 2003;348:2609–17.
Neben K, Jauch A, Bertsch U, Heiss C, Hielscher T, Seckinger A, et al. Combining information regarding chromosomal aberrations t(4;14) and del(17p13) with the International Staging System classification allows stratification of myeloma patients undergoing autologous stem cell transplantation. Haematologica. 2010;95:1150–7.
van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat softw. 2011;45:1–67.
Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–42.
Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau J-L, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–32.
Stadtmauer EA, Weber DM, Niesvizky R, Belch A, Prince MH, San Miguel JF, et al. Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma. Eur J Haematol. 2009;82:426–32.
Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37.
Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet. 2007;370:1209–18.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2014;372:141206080130007.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–31.
Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral Ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–34.
Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–91.
McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–81.
McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279–89.
Acknowledgements
The trial was designed and conducted by the German Myeloma Multicenter Group (GMMG). We thank all investigators, study nurses, research staff, the coordination centers for clinical trials (KKS) in Heidelberg and Leipzig, and—most importantly—the participating patients and their families. Furthermore we thank the Dietmar Hopp-Stiftung, Celgene, Chugai, and Amgen for their support of the trial.
Author information
Authors and Affiliations
Consortia
Contributions
HG designed the trial and HG, MAB, JS, NB, CH and TH analyzed the data. HG, MAB, JS, MSR, JH, AJ, PB, MG, SK, MS-H, PR, UG, RF, MH, HM, HWL, CS, AN, HS, RN, and KW collected data. MAB wrote and all co-authors revised and approved the article.
Corresponding author
Ethics declarations
Conflict of interest
HG—Amgen: consultancy, research funding; Novartis: honoraria, research funding; ArtTempi: honoraria; Janssen: consultancy, honoraria, research funding; Sanofi: consultancy, research funding; Mundipharma: research funding; Takeda: consultancy, research funding; Celgene: consultancy, honoraria, research funding; Bristol-Myers Squibb: consultancy, honoraria, research funding; Adaptive Biotechnology: consultancy; Chugai: honoraria, research funding. MAB—Takeda: consultancy, honoraria; Novartis: consultancy, research funding. Travel support: Celgene, Amgen, and Janssen. M-SB: Celgene: consultancy, honoraria; Novartis: consultancy, honoraria, research funding; BMS: consultancy, honoraria, research funding; Amgen: consultancy, honoraria, research funding. JH—Janssen: honoraria, advisory board; Amgen: advisory board; BMS: honoraria, advisory board, research funding; Oncotracker: advisory board; Adaptive Biotech: advisory board; GSK: advisory board. Celgene: consultancy, honoraria, Other: advisory board, research funding. CM-T—research funding: Pfizer, Daiichi Sankyo, BiolineRx, Bayer; advisory boards: Pfizer, Janssen. MS-H—consultancy: Celgene; financial support of educational meetings: Janssen, Takeda, Novartis, Pfizer, Roche, Vifor, Celgene. PR—Honoraria: Takeda, BMS, Roche, Celgene, Sanofi-Aventis; travel support: Celgene, Takeda, Abbvie. UG—honoraria: Sirtex, Daiichi Sankyo, Boehringer Ingelheim, Amgen, Servier, AstraZeneca; consultancy, advisory boards: Merck, BMS, Hexal, Amgen, Celgene, Johnson & Johnson, MSD; travel support: Merck, Amgen, Boehringer Ingelheim. RF—Takeda: honoraria; Bristol-Meyers Squibb: honoraria, Other: travel grant; Celgene: honoraria, Other: travel grant, research funding; Janssen: honoraria; Amgen: honoraria. MH—Novartis: honoraria; Roche: honoraria; Amgen: honoraria; Takeda: honoraria. CS—Novartis: honoraria, research funding; Takeda: honoraria, research funding; Janssen: honoraria, research funding; Celgene: honoraria; BMS: honoraria; Amgen: honoraria; GSK: honoraria. AN—honoraria: Celgene, Takeda, Amgen, Alexion, Sanofi, Janssen, BMS. Research funding: Celgene, Janssen, Takeda. Travel support: Celgene, Takeda, Alexion. HS—Celgene: honoraria, Other: travel suppport, research funding; Janssen: honoraria, Other: travel support, research funding; Novartis: honoraria, Other: travel suppport, research funding; Takeda: honoraria; Amgen: honoraria, Other: travel suppport, research funding; Bristol-Myers Squibb: honoraria, Other: travel suppport, research funding. BB—Janssen: honoraria. KW—Amgen, Celgene, Janssen, and Sanofi: research funding; Amgen, BMS, Celgene, Janssen, Takeda, Adaptive Biotech: honoraria; Amgen, Adaptive Biotech, BMS, Celgene, Janssen, Juno, Sanofi, GSK, Karyopharm and Takeda: consultancy, membership on an entity’s Board of Directors or advisory committees.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Goldschmidt, H., Baertsch, MA., Schlenzka, J. et al. Salvage autologous transplant and lenalidomide maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: the randomized GMMG phase III trial ReLApsE. Leukemia 35, 1134–1144 (2021). https://doi.org/10.1038/s41375-020-0948-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0948-0
This article is cited by
-
Health-related quality of life and quality-adjusted progression free survival for carfilzomib and dexamethasone maintenance following salvage autologous stem-cell transplantation in patients with multiple myeloma: a randomized phase 2 trial by the Nordic Myeloma Study Group
Journal of Patient-Reported Outcomes (2024)
-
Treatment pattern and outcomes of re-induction therapy prior to stem cell transplantation in patients with relapsed/refractory multiple myeloma in Germany
Bone Marrow Transplantation (2024)
-
Bridging advanced myeloma patients to subsequent treatments and clinical trials with classical chemotherapy and stem cell support
Bone Marrow Transplantation (2023)
-
Non-interventional Study Evaluating the Mobilization of Stem Cells by Plerixafor Before Salvage Autologous Stem Cell Transplant in Relapsed Multiple Myeloma (IFM-2015-03)
Clinical Hematology International (2023)
-
Carfilzomib, lenalidomide and dexamethasone followed by a second ASCT is an effective strategy in first relapse multiple myeloma: a study on behalf of the Chronic malignancies working party of the EBMT
Bone Marrow Transplantation (2023)