Abstract
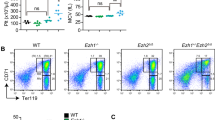
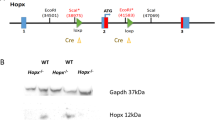
FZR1 has been implicated as a master regulator of the cell cycle and quiescence, but its roles and molecular mechanisms in the pathogenesis of severe aplastic anemia (SAA) are unclear. Here, we report that FZR1 is downregulated in SAA HSCs compared with healthy control and is associated with decreased quiescence of HSC. Haploinsufficiency of Fzr1 shows impaired quiescence and self-renewal ability of HSC in two Fzr1 heterozygous knockout mouse models. Mechanistically, FZR1 insufficiency inhibits the ubiquitination of RUNX1 protein at lysine 125, leading to the accumulation of RUNX1 protein, which disturbs the quiescence of HSCs in SAA patients. Moreover, downregulation of Runx1 reversed the loss of quiescence and impaired long-term self-renew ability in Fzr1+/− HSCs in vivo and impaired repopulation capacity in BM from SAA patients in vitro. Our findings, therefore, raise the possibility of a decisive role of the FZR1-RUNX1 pathway in the pathogenesis of SAA via deregulation of HSC quiescence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Young NS. Aplastic anemia. N Engl J Med. 2018;379:1643–56. https://doi.org/10.1056/NEJMra1413485.
Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. A severe and consistent deficit in marrow and circulating primitive hematopoietic cells (long-term culture-initiating cells) in acquired aplastic anemia. Blood. 1996;88:1983–91.
Schoettler ML, Nathan DG. The pathophysiology of acquired aplastic anemia: current concepts revisited. Hematol Oncol Clin North Am. 2018;32:581–94. https://doi.org/10.1016/j.hoc.2018.03.001.
Laurenti E, Frelin C, Xie S, Ferrari R, Dunant CF, Zandi S, et al. CDK6 levels regulate quiescence exit in human hematopoietic stem cells. Cell Stem Cell. 2015;16:302–13. https://doi.org/10.1016/j.stem.2015.01.017.
Kaschutnig P, Bogeska R, Walter D, Lier A, Huntscha S, Milsom MD. The Fanconi anemia pathway is required for efficient repair of stress-induced DNA damage in haematopoietic stem cells. Cell Cycle. 2015;14:2734–42. https://doi.org/10.1080/15384101.2015.1068474.
Wan L, Chen M, Cao J, Dai X, Yin Q, Zhang J, et al. The APC/C E3 ligase complex activator FZR1 restricts BRAF oncogenic function. Cancer Discov. 2017;7:424–41. https://doi.org/10.1158/2159-8290.CD-16-0647.
Zhao X, Gao S, Wu Z, Kajigaya S, Feng X, Liu Q, et al. Single-cell RNA-seq reveals a distinct transcriptome signature of aneuploid hematopoietic cells. Blood. 2017;130:2762–73. https://doi.org/10.1182/blood-2017-08-803353.
Cappell SD, Chung M, Jaimovich A, Spencer SL, Meyer T. Irreversible APC(Cdh1) inactivation underlies the point of no return for cell-cycle entry. Cell. 2016;166:167–80. https://doi.org/10.1016/j.cell.2016.05.077.
Cappell SD, Mark KG, Garbett D, Pack LR, Rape M, Meyer T. EMI1 switches from being a substrate to an inhibitor of APC/C(CDH1) to start the cell cycle. Nature. 2018;558:313–7. https://doi.org/10.1038/s41586-018-0199-7.
Ly PT, Wang H. Fzr/Cdh1 promotes the differentiation of neural stem cell lineages in drosophila. Front Cell Dev Biol. 2020;8:60 https://doi.org/10.3389/fcell.2020.00060.
Mao DD, Gujar AD, Mahlokozera T, Chen I, Pan Y, Luo J, et al. A CDC20-APC/SOX2 signaling axis regulates human glioblastoma stem-like cells. Cell Rep. 2015;11:1809–21. https://doi.org/10.1016/j.celrep.2015.05.027.
De K, Grubb TM, Zalenski AA, Pfaff KE, Pal D, Majumder S, et al. Hyperphosphorylation of CDH1 in glioblastoma cancer stem cells attenuates APC/C(CDH1) activity and pharmacologic inhibition of APC/C(CDH1/CDC20) compromises viability. Mol Cancer Res. 2019;17:1519–30. https://doi.org/10.1158/1541-7786.MCR-18-1361.
Camitta BM, Thomas ED, Nathan DG, Gale RP, Kopecky KJ, Rappeport JM, et al. A prospective study of androgens and bone marrow transplantation for treatment of severe aplastic anemia. Blood. 1979;53:504–14.
Chen J, Suo S, Tam PP, Han JJ, Peng G, Jing N. Spatial transcriptomic analysis of cryosectioned tissue samples with Geo-seq. Nat Protoc. 2017;12:566–80. https://doi.org/10.1038/nprot.2017.003.
Killick SB, Cox CV, Marsh JC, Gordon-Smith EC, Gibson FM. Mechanisms of bone marrow progenitor cell apoptosis in aplastic anaemia and the effect of anti-thymocyte globulin: examination of the role of the Fas-Fas-L interaction. Br J Haematol. 2000;111:1164–9. https://doi.org/10.1046/j.1365-2141.2000.02485.x.
Liu H, Wu F, Jiang S. [Induction of apoptosis of CD34+ cells by serum from two patients with severe aplastic anemia]. Zhonghua Xue Ye Xue Za Zhi. 1998;19:514–7.
Yao W, Qian W, Zhu C, Gui L, Qiu J, Zhang C. Cdh1-APC is involved in the differentiation of neural stem cells into neurons. Neuroreport. 2010;21:39–44. https://doi.org/10.1097/WNR.0b013e32833312fe.
Eguren M, Porlan E, Manchado E, Garcia-Higuera I, Canamero M, Farinas I, et al. The APC/C cofactor Cdh1 prevents replicative stress and p53-dependent cell death in neural progenitors. Nat Commun. 2013;4:2880 https://doi.org/10.1038/ncomms3880.
Garcia-Higuera I, Manchado E, Dubus P, Canamero M, Mendez J, Moreno S, et al. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat Cell Biol. 2008;10:802–11. https://doi.org/10.1038/ncb1742.
Naoe H, Chiyoda T, Ishizawa J, Masuda K, Saya H, Kuninaka S. The APC/C activator Cdh1 regulates the G2/M transition during differentiation of placental trophoblast stem cells. Biochem Biophys Res Commun. 2013;430:757–62. https://doi.org/10.1016/j.bbrc.2012.11.075.
Zhou Y, Ching YP, Chun AC, Jin DY. Nuclear localization of the cell cycle regulator CDH1 and its regulation by phosphorylation. J Biol Chem. 2003;278:12530–6. https://doi.org/10.1074/jbc.M212853200.
Zhou Y, Ching YP, Ng RW, Jin DY. Differential expression, localization and activity of two alternatively spliced isoforms of human APC regulator CDH1. Biochem J. 2003;374:349–58. https://doi.org/10.1042/BJ20030600.
Holt JE, Weaver J, Jones KT. Spatial regulation of APCCdh1-induced cyclin B1 degradation maintains G2 arrest in mouse oocytes. Development. 2010;137:1297–304. https://doi.org/10.1242/dev.047555.
Seah MK, Holt JE, Garcia-Higuera I, Moreno S, Jones KT. The APC activator fizzy-related-1 (FZR1) is needed for preimplantation mouse embryo development. J Cell Sci. 2012;125:6030–7. https://doi.org/10.1242/jcs.110155.
Chen Z, Huo D, Li L, Liu Z, Li Z, Xu S, et al. Nuclear DEK preserves hematopoietic stem cells potential via NCoR1/HDAC3-Akt1/2-mTOR axis. J Exp Med. 2021;218, https://doi.org/10.1084/jem.20201974.
Lin FC, Karwan M, Saleh B, Hodge DL, Chan T, Boelte KC, et al. IFN-gamma causes aplastic anemia by altering hematopoietic stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699–708. https://doi.org/10.1182/blood-2014-01-549527.
Lerner C, Harrison DE. 5-Fluorouracil spares hemopoietic stem cells responsible for long-term repopulation. Exp Hematol. 1990;18:114–8.
Ramanauskaite G, Vaitkuviene A, Kaseta V, Vitlipaite A, Liubaviciute A, Biziuleviciene G. Bone marrow-derived lineage-negative cells accelerate skin regeneration in vivo. Turk J Biol. 2018;42:205–12. https://doi.org/10.3906/biy-1711-91.
Zaro, BW, Noh, JJ, Mascetti, VL, Demeter, J, George, B, Zukowska, M, et al. Proteomic analysis of young and old mouse hematopoietic stem cells and their progenitors reveals post-transcriptional regulation in stem cells. Elife. 2020;9, https://doi.org/10.7554/eLife.62210.
Biggs JR, Peterson LF, Zhang Y, Kraft AS, Zhang DE. AML1/RUNX1 phosphorylation by cyclin-dependent kinases regulates the degradation of AML1/RUNX1 by the anaphase-promoting complex. Mol Cell Biol. 2006;26:7420–9. https://doi.org/10.1128/MCB.00597-06.
Cai X, Gao L, Teng L, Ge J, Oo ZM, Kumar AR, et al. Runx1 deficiency decreases ribosome biogenesis and confers stress resistance to hematopoietic stem and progenitor cells. Cell Stem Cell. 2015;17:165–77. https://doi.org/10.1016/j.stem.2015.06.002.
Sato T, Kim S, Selleri C, Young NS, Maciejewski JP. Measurement of secondary colony formation after 5 weeks in long-term cultures in patients with myelodysplastic syndrome. Leukemia. 1998;12:1187–94. https://doi.org/10.1038/sj.leu.2401084.
Rizzo S, Scopes J, Draycott GS, Pocock C, Foukaneli T, Rutherford TR, et al. Quiescent (5-fluorouracil-resistant) aplastic anemia hematopoietic cells in vitro. Exp Hematol. 2004;32:665–72. https://doi.org/10.1016/j.exphem.2004.04.003.
Li X, Plett PA, Yang Y, Hong P, Freie B, Srour EF, et al. Fanconi anemia type C-deficient hematopoietic stem/progenitor cells exhibit aberrant cell cycle control. Blood. 2003;102:2081–4. https://doi.org/10.1182/blood-2003-02-0536.
Barroca V, Mouthon MA, Lewandowski D, Brunet de la Grange P, Gauthier LR, Pflumio F, et al. Impaired functionality and homing of Fancg-deficient hematopoietic stem cells. Hum Mol Genet. 2012;21:121–35. https://doi.org/10.1093/hmg/ddr447.
Ishizawa J, Kuninaka S, Sugihara E, Naoe H, Kobayashi Y, Chiyoda T, et al. The cell cycle regulator Cdh1 controls the pool sizes of hematopoietic stem cells and mature lineage progenitors by protecting from genotoxic stress. Cancer Sci. 2011;102:967–74. https://doi.org/10.1111/j.1349-7006.2011.01884.x.
Ewerth D, Kreutmair S, Schmidts A, Ihorst G, Follo M, Wider D, et al. APC/C(Cdh1) regulates the balance between maintenance and differentiation of hematopoietic stem and progenitor cells. Cell Mol Life Sci. 2019;76:369–80. https://doi.org/10.1007/s00018-018-2952-3.
The I, Ruijtenberg S, Bouchet BP, Cristobal A, Prinsen MB, van Mourik T, et al. Rb and FZR1/Cdh1 determine CDK4/6-cyclin D requirement in C. elegans and human cancer cells. Nat Commun. 2015;6:5906 https://doi.org/10.1038/ncomms6906.
Yoshizato T, Dumitriu B, Hosokawa K, Makishima H, Yoshida K, Townsley D, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35–47. https://doi.org/10.1056/NEJMoa1414799.
Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337–47. https://doi.org/10.1182/blood-2016-01-636381.
Albitar A, Townsley D, Ma WL, De Dios I, Funari V, Young NS, et al. Higher mutation rate in patients with aplastic anemia using peripheral blood cfDNA as compared with bone marrow cells. Blood. 2016;128, https://doi.org/10.1182/blood.V128.22.3902.3902.
Ichikawa M, Goyama S, Asai T, Kawazu M, Nakagawa M, Takeshita M, et al. AML1/Runx1 negatively regulates quiescent hematopoietic stem cells in adult hematopoiesis. J Immunol. 2008;180:4402–8. https://doi.org/10.4049/jimmunol.180.7.4402.
Cai X, Gaudet JJ, Mangan JK, Chen MJ, De Obaldia ME, Oo Z, et al. Runx1 loss minimally impacts long-term hematopoietic stem cells. PLoS One. 2011;6:e28430 https://doi.org/10.1371/journal.pone.0028430.
Zhao X, Chen A, Yan X, Zhang Y, He F, Hayashi Y, et al. Downregulation of RUNX1/CBFbeta by MLL fusion proteins enhances hematopoietic stem cell self-renewal. Blood. 2014;123:1729–38. https://doi.org/10.1182/blood-2013-03-489575.
Jacob B, Osato M, Yamashita N, Wang CQ, Taniuchi I, Littman DR, et al. Stem cell exhaustion due to Runx1 deficiency is prevented by Evi5 activation in leukemogenesis. Blood. 2010;115:1610–20. https://doi.org/10.1182/blood-2009-07-232249.
Challen GA, Goodell MA. Runx1 isoforms show differential expression patterns during hematopoietic development but have similar functional effects in adult hematopoietic stem cells. Exp Hematol. 2010;38:403–16. https://doi.org/10.1016/j.exphem.2010.02.011.
Tsuzuki S, Hong D, Gupta R, Matsuo K, Seto M, Enver T. Isoform-specific potentiation of stem and progenitor cell engraftment by AML1/RUNX1. PLoS Med. 2007;4:e172 https://doi.org/10.1371/journal.pmed.0040172.
Nakahata T, Ogawa M. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc Natl Acad Sci USA. 1982;79:3843–7. https://doi.org/10.1073/pnas.79.12.3843.
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2017YFC1001903, 2017YFA0106700), National Science Foundation of China (81970100), China Postdoctoral Science Foundation (2019M663977), Chongqing Postdoctoral Science Foundation (4142Z2373), and Army Major Scientific Research Projects (AWS17J007).
Author information
Authors and Affiliations
Contributions
CFZ designed and performed the experiments, analyzed data, and wrote the manuscript; MK supervised the experiments conducted in the laboratories; ZLL analyzed data; XQJ, ZC, YYL, WRW, LM, contributed to the relevant discussions; JPC conceived the project; and YH conceived, designed, and supervised the experiments, analyzed results, and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhou, C., Kuang, M., Liu, Z. et al. Insufficiency of FZR1 disturbs HSC quiescence by inhibiting ubiquitin-dependent degradation of RUNX1 in aplastic anemia. Leukemia 36, 834–846 (2022). https://doi.org/10.1038/s41375-021-01445-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01445-5
This article is cited by
-
Functions and regulatory mechanisms of resting hematopoietic stem cells: a promising targeted therapeutic strategy
Stem Cell Research & Therapy (2023)
-
USP10 deubiquitinates RUNX1 and promotes proneural-to-mesenchymal transition in glioblastoma
Cell Death & Disease (2023)