Abstract
Advanced systemic mastocytosis (AdvSM) is a rare myeloid neoplasm associated with poor overall survival (OS). This study (NCT04695431) compared clinical outcomes between patients with AdvSM treated with avapritinib in the Phase 1 EXPLORER (NCT0256198) and Phase 2 PATHFINDER (NCT03580655) trials (N = 176) and patients treated with best available therapy (BAT; N = 141). A multi-center, observational, retrospective chart review study was conducted at six study sites (four European, two American) to collect data from patients with AdvSM who received BAT; these data were pooled with data from EXPLORER and PATHFINDER. Comparisons between outcomes of OS, duration of treatment (DOT), and maximum reduction in serum tryptase were conducted between the treatment cohorts, with adjustment for key covariates. The results indicated that the avapritinib cohort had significantly better survival (adjusted hazard ratio (HR) (95% confidence interval (CI)): 0.48 (0.29, 0.79); p = 0.004) and significantly longer DOT (HR: 0.36 (0.26, 0.51); p < 0.001) compared to the BAT cohort. Additionally, the mean difference in percentage maximum reduction in serum tryptase levels was 60.3% greater in the avapritinib cohort (95% CI: −72.8, −47.9; p < 0.001). With no randomized controlled trials comparing avapritinib to BAT, these data offer crucial insights into the improved efficacy of avapritinib for the treatment of AdvSM.
Similar content being viewed by others
Introduction
Advanced systemic mastocytosis (AdvSM) is a rare myeloid neoplasm characterized by accumulation of neoplastic mast cells in various tissues and organs [1,2,3,4], often leading to progressive organ damage, mainly manifesting as cytopenias of one or more hematopoietic lineage(s) and dysfunction of gastrointestinal organs [5]. The World Health Organization defines three subtypes of AdvSM: aggressive systemic mastocytosis (ASM), SM with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL) [6]. Patients with AdvSM have a poor prognosis, with a median overall survival (OS) of ~3.5 years for ASM, 2 years for SM-AHN, and 0.5–2 years for MCL [7,8,9,10].
As the majority (>90%) of patients with AdvSM carry a KIT D816V mutation [11], recent therapeutic advances have focused on KIT inhibitors [12]. Treatment options for patients with AdvSM include the multikinase KIT inhibitor midostaurin, for which efficacy and safety has been reported in several clinical trials and observational studies [9, 13,14,15,16,17,18]. In addition, imatinib is a treatment option for the limited indication of ASM patients who are KIT D816V-negative or with unknown KIT mutation status [19]. Commonly used off-label cytoreductive therapies include cladribine [13, 20,21,22,23] and interferon alfa [22, 24]. For treatment-resistant patients and those with rapidly progressive disease after tyrosine kinase inhibitor (TKI) treatment, multiagent chemotherapy and subsequent allogenic hematopoietic stem cell transplantation (HSCT) are considerations [25]. Indeed, HSCT is the only established curative treatment option for these patients.
Avapritinib, a highly selective and potent inhibitor of D816V-mutated KIT, was evaluated in adults with centrally confirmed AdvSM in two multi-center, single-arm, open-label clinical trials, the Phase I EXPLORER trial (ClinicalTrials.gov Identifier: NCT02561988) [26] and Phase II PATHFINDER trial (NCT03580655) [27]. Analysis of data from 69 patients with AdvSM in EXPLORER reported an estimated 24-month OS rate of 76% (95% confidence interval (CI), 64–87%) and that 99% of patients achieved ≥50% reduction from baseline in serum tryptase (a common marker of mast cell activation) [26]. Similarly, in a pre-specified interim analysis of 62 patients who received avapritinib primarily at a starting dose of 200 mg daily from PATHFINDER, the estimated 12-month OS rate was 86% and 93% of patients achieved ≥50% reduction from baseline in serum tryptase [27].
Given the single-arm design of EXPLORER and PATHFINDER, a direct comparison of avapritinib to alternative therapies for AdvSM is not feasible in the context of a controlled clinical trial. However, comparing the efficacy of avapritinib with that of existing therapies for AdvSM is essential to inform clinical decision making. To address this need, the current study compared the efficacy of avapritinib to a real-world cohort of similar patients receiving best available therapy (BAT) for AdvSM.
Methods
Study population
Clinical trial data (avapritinib cohort)
Individual patient data as of the April 20, 2021, data cut-off from the Phase I EXPLORER and Phase II PATHFINDER trials (data on file, Blueprint Medicines Corporation) were used in this analysis. In EXPLORER, the starting dose of avapritinib was escalated from 30 to 400 mg daily while in PATHFINDER, all but two patients received 200 mg daily.
Real-world data (BAT cohort)
A multi-center, observational, retrospective chart review study was conducted to generate real-world data on BAT for AdvSM. Longitudinal, individual-level data were collected via medical chart abstraction on eligible patients with AdvSM who received systemic treatment at the following Centers of Excellence for the treatment of AdvSM: Dana-Farber Cancer Institute (United States [US]), Guy’s and St Thomas’ NHS Foundation Trust (United Kingdom), Hospital Virgen del Valle (Spain), Medical University of Vienna (Austria), University Hospital Mannheim (Germany), and the Stanford Cancer Institute (US). De-identified data from eligible patients at these sites were abstracted from medical records into a standardized, structured, electronic case report form from March 26, 2021, to October 4, 2021. Site research personnel were provided training on the study protocol and case report form, and data collection was followed by a query resolution process. Ethics Committee approvals were gained at each study site.
Sample selection
Patients receiving treatment with BAT for AdvSM were identified based on inclusion and exclusion criteria similar to those from EXPLORER and PATHFINDER (full list of criteria provided in Supplementary Table 1). Adults (aged ≥ 18 years) with a diagnosis of AdvSM and documented subtype in their chart (ASM, SM-AHN, or MCL), and who had received ≥1 line of systemic therapy (not necessarily as first line (1L)) for AdvSM at a participating site on or after January 1, 2009, were included. If a patient received multiple lines of therapy at a participating site, data on all available therapies were collected and analyzed (i.e., the patient could contribute more than one line of therapy to the analysis). The date of initiation of each line of therapy at the participating site was defined as the index date.
Patients in the BAT cohort were excluded if they had a history of another primary malignancy that was diagnosed or required therapy within 3 years before the index date, except for completely resected basal cell and squamous cell skin cancer, curatively treated localized prostate cancer, and completely resected carcinoma in situ at any site, or if they received avapritinib as the first therapy for AdvSM.
Study endpoints
The primary endpoint was OS, defined for the BAT cohort as the time interval between initiation of each line of therapy and death due to any cause, and for the avapritinib cohort as the time interval between the first dose of avapritinib and death due to any cause. If alive at study end, patients were censored at the date of last contact (BAT cohort), or at the last known date alive (avapritinib cohort). Secondary endpoints included (1) duration of therapy (DOT), defined as the time interval between initiation of each therapy to discontinuation for any reason; (2) change in serum tryptase levels from baseline to 2 months (to correspond to the day 1 of cycle 3 assessment of serum tryptase in EXPLORER and PATHFINDER and to maximize the sample size), and (3) maximum reduction in serum tryptase levels from baseline. Other response endpoints such as complete and partial response and clinical improvement were not included due to lack of uniform assessment criteria in non-protocol clinical practice. Adverse events (AEs) that resulted in treatment modification or discontinuation, hospitalization, or death according to the responsible physician’s evaluation were reported for the BAT cohort only, as comparable definitions of AEs were not available in EXPLORER and PATHFINDER.
Baseline covariates
Multiple prognostic factors for survival and clinical outcomes in patients with AdvSM, as well as confounders for the effect of treatment on outcomes, were considered [12,13,14, 16, 28, 29]. These a priori-defined key adjustment covariates, informed through clinical input as well as prognostic scores such as the mutation-adjusted risk score [14] and the International Prognostic Scoring System in mastocytosis [30], included age; sex; region (North America or Europe); European Cooperative Oncology Group performance status score; AdvSM subtype (SM-AHN, ASM, or MCL, assessed at the last diagnosis evaluation prior to or on the initiation of an included line of therapy); presence of skin involvement (including reported mastocytosis in the skin or urticaria pigmentosa); number and types of prior lines received (TKI, cytoreductive, or biologic or other systemic therapy); presence of anemia (hemoglobin <10 g/dl), thrombocytopenia (platelet count <100 × 109/l), or leukocyte count ≥16 × 109/l; serum tryptase level ≥125 ng/ml; and presence and number of mutations within the SRSF2/ASXL1/RUNX1 gene panel [14, 30].
Statistical analyses
AEs were evaluated in all BAT patients meeting the above inclusion and exclusion criteria. In comparative analyses of the primary and secondary efficacy endpoints, BAT patients were excluded if they had missing data on a key adjustment covariate. Patients in the safety populations of EXPLORER and PATHFINDER (i.e., received at least one dose of avapritinib and had confirmed AdvSM subtype based on adjudication by the trial Response Assessment Committee (RAC)) were included in the comparative analyses. RAC-response evaluable (RAC-RE) patients from PATHFINDER were included in one subgroup analysis. An integrated dataset containing patient-level data from the avapritinib and BAT cohorts was created, with harmonization between the definitions of outcomes and key covariates.
Cohort characteristics and covariates
Descriptive analyses were used to summarize therapies received by patients in the BAT cohort, as well as key covariates in both cohorts. Means, standard deviations (SDs), and medians with ranges were reported for continuous variables; frequencies and proportions were reported for categorical variables. Comparisons between cohorts were conducted using the Wilcoxon rank-sum test for continuous variables and chi-squared test for categorical variables.
Efficacy analyses
For OS and DOT, the median time-to-event, corresponding 95% CI, and log-rank test p values were reported. Unadjusted survival and on-treatment rates at specific time points were obtained using the Nelson–Aalen estimator [31, 32], and unadjusted OS and DOT estimates up to each of these timepoints were obtained using the Kaplan–Meier method.
Comparative analyses of OS, DOT, and change in serum tryptase levels employed a two-step process to obtain an effect estimate that was doubly robust against confounding [33]. First, stabilized inverse-probability-of-treatment-weights (IPTW) were created using logistic regression models, calculated as the inverse of the propensity score, i.e., probability of being in the respective treatment cohort (i.e., avapritinib or BAT), conditional on pre-specified key covariates. Weights were truncated at the 1st and 99th percentiles to reduce variability. Standardized differences were used to assess balance of covariates before and after IPTW weighting, with a difference >10% indicating meaningful imbalance between the two cohorts [34].
Next, IPTW-weighted multivariable Cox proportional hazards models were used to compare survival and DOT and IPTW-weighted multivariable generalized estimating equation linear models were used to compare change in serum tryptase between the avapritinib and BAT cohorts, with further adjustment for key covariates that remained unbalanced after weighting. A two-sided p < 0.05 was considered statistically significant without multiplicity adjustment. Robust variance estimation was used to account for the within-subject correlation of BAT cohort patients who contributed multiple lines of therapy, as well as for the application of weights.
Safety analyses
AEs that resulted in treatment modification or discontinuation, hospitalization, or death were summarized using descriptive statistics for the BAT cohort.
Subgroup and sensitivity analyses
The primary endpoint of OS was compared in the following subgroups: (1) patients who initiated 1L avapritinib at any dose in EXPLORER and PATHFINDER vs. patients who received 1L BAT; (2) all patients who initiated avapritinib at ≤200 mg in EXPLORER and PATHFINDER vs. BAT regardless of the number of prior lines of therapy; (3) patients who received at least one prior systemic therapy (2L + patients) prior to initiating avapritinib at ≤200 mg in EXPLORER and PATHFINDER vs. 2L + BAT patients; (4) 2L + patients who started avapritinib at 200 mg in EXPLORER and PATHFINDER vs. 2L + patients who received BAT; and (5) 2L + patients who started avapritinib at 200 mg in PATHFINDER only vs. 2L + patients who received BAT, using the PATHFINDER safety population and RAC-RE population, respectively. In sensitivity analyses of OS, the impact of excluding patients with missing performance status was evaluated, and index year of treatment was included as a covariate in the Cox model to assess the impact of trends over time in AdvSM care (it was not included in the IPTW model due to inadequate overlap in index year between the avapritinib and BAT cohorts).
Software
All data cleaning and analyses were conducted using SAS® Enterprise Guide® (version 7.1) and R (version 3.6.3).
Results
Study sample
Data were collected from 161 patients who received BAT for AdvSM. After excluding 20 (12.4%) patients with missing performance status, 141 were included in the BAT cohort for comparison with 176 patients in the avapritinib cohort enrolled in EXPLORER (n = 69) and PATHFINDER (n = 107). While patients in the avapritinib cohort contributed data on a single line of therapy each, the 141 BAT patients contributed 222 lines of therapy. The median number of lines of therapy per BAT patient was 1.0 (range, 1.0–7.0) (Table 1). Of the 222 lines of therapy, 118 (53.2%) were first, 69 (31.1%) second, and 35 (15.8%) third line or later. Across lines, patients were most frequently treated with TKIs (54.1% of lines), followed by cytoreductive therapy (41.0%) and biologic therapies (11.3%). Among 196 lines of therapy with agent-level information available, midostaurin (50.5%) and cladribine (25.0%) were most often used.
Baseline characteristics and IPTW weighting
Prior to weighting, region, presence of thrombocytopenia, AdvSM subtype, serum tryptase level ≥125 ng/ml, presence and number of mutated genes within SRSF2/ASXL1/RUNX1 gene panel, number of prior lines of therapy, and having received prior TKI therapy were unbalanced between the avapritinib and BAT cohorts (Table 2). The truncated stabilized IPTW weights calculated based on the key baseline covariates had a mean of 0.96 (SD: 0.71; range: 0.46–4.45), indicating the IPTW model was appropriate and stable (Supplementary Table 2). After weighting by truncated stabilized IPTW weights, standardized differences decreased to <10% for most covariates, indicating the two cohorts were more comparable with regards to key covariates (Supplementary Table 3).
Main analysis
Overall survival
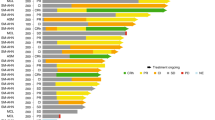
In the unweighted sample, there were 34 (19.3%) deaths among 176 avapritinib patients and 84 (59.6%) deaths among 141 BAT patients (Table 3), with a mean follow-up of 17.9 and 25.7 months, respectively. Median OS was not reached (95% CI: 46.9, not estimable) for the avapritinib cohort and was 23.4 (19.5, 32.6) months for the BAT cohort (log-rank p < 0.001; Fig. 1a). In adjusted analysis, after weighting, and further adjustment for variables with standardized difference >10% after weighting, OS remained significantly longer in the avapritinib cohort compared with the BAT cohort (IPTW-weighted median OS (95% CI) avapritinib vs. BAT: 49.0 (46.9, not estimable) vs. 26.8 (18.2, 39.7) months; HR (95% CI): 0.48 (0.29, 0.79); p = 0.004). The IPTW-weighted OS rates were higher for the avapritinib cohort relative to the BAT cohort across all time points (e.g., 6 months: 96.4% vs. 84.8%; 12 months: 86.4% vs. 73.8%; 24 months: 74.6% vs. 50.9%; 36 months: 68.0% vs. 42.7%) (Table 3). Survival was significantly longer for the avapritinib cohort at all time points tested (p < 0.05 for 6 months and subsequent time points), except at 3 months (p = 0.087).
The three panels refer to OS among the overall population (a), OS among patients treated with 2L+ therapy (b), and DOT among the overall population (c). 2L+ second or later line of therapy, AdvSM advanced systemic mastocytosis, BAT best available therapy, DOT duration of therapy, OS overall survival. aAll Kaplan–Meier curves were truncated at the maximum follow-up of the avapritinib cohort. bA total of 222 lines of therapy were contributed by 141 patients in the BAT cohort. cIn the subgroup analysis comparing avapritinib patients treated at ≤200 mg to BAT patients in 2L+, a total of 104 lines of therapy were contributed by 73 patients in BAT cohort. dA total of 213 lines of therapy were contributed by 137 patients in the BAT cohort. Lines of therapy with unknown discontinuation date and unknown last known prescription date were excluded from the analysis of duration of treatment.
In the sensitivity analysis, OS was not significantly different between the BAT efficacy analysis sample (i.e., baseline performance status available prior to initiation in each line of therapy (N = 222, 88.8% of 250 prior lines of therapy)) and the BAT full sample with missing baseline performance status (N = 250 lines of therapy) (log-rank p = 0.33). Unweighted Kaplan–Meier analysis suggested that OS was significantly improved in the avapritinib cohort compared to the full BAT sample (including patients with missing baseline performance scores) (log-rank p < 0.001), consistent with the main analysis. In addition, when the main analysis was performed with an indicator for index year as a covariate in the Cox regression model for OS, the results were not different from the main analysis (HR (95% CI): 0.48 (0.27, 0.85); p = 0.01).
Duration of treatment
The DOT analyses included 176 patients in the avapritinib cohort and 137 patients in the BAT cohort contributing 213 lines of therapy (Table 4 and Fig. 1c). In the unweighted sample, the median DOT was 30.6 (95% CI: 21.4, not estimable) months in the avapritinib cohort and 5.5 (5.1, 7.0) months in the BAT cohort. In the adjusted analysis, DOT remained significantly longer in the avapritinib cohort (IPTW-weighted median (95% CI) avapritinib vs. BAT: 23.8 (20.3, 40.9) vs. 5.4 (5.0, 7.5) months; HR (95% CI): 0.36 (0.26, 0.51); p < 0.001). After weighting, the proportion of patients that stayed on treatment was significantly higher for the avapritinib cohort than the BAT cohort across all time points (e.g., 6 months: 85.6% vs. 45.0%; 12 months: 67.7% vs. 32.5%; 18 months: 61.3% vs. 20.4%; all p < 0.001).
Change in serum tryptase levels
The analysis of 2-month changes in serum tryptase levels included 154 patients in the avapritinib cohort and 43 patients in the BAT cohort (Table 5). The mean percentage change in serum tryptase level from baseline to 2 months was greater for the avapritinib cohort (−71.5% (SD: 35.9%)) than the BAT cohort (37.9% (SD: 269.3%)) (mean difference: −103.0% (95% CI: −167.1%, −38.9%); p = 0.002). The same trend was observed after weighting, with a greater mean percentage change in serum tryptase level in the avapritinib cohort (−71.3% (SD: 35.2%)) compared to the BAT cohort (1.7% (148.8%)), although the corresponding mean difference in the percentage change was not estimable by an adjusted linear model due to model non-convergence caused by the small sample size of the BAT cohort.
The analysis of maximum reduction in serum tryptase included 175 patients in the avapritinib cohort and 116 patients in the BAT cohort (Table 5). In the unweighted sample, the maximum percentage reduction of serum tryptase level was −86.6% (SD: 18.2%) in the avapritinib cohort and −9.2% (161.4%) in the BAT cohort, corresponding to an unadjusted mean difference of −77.9% (95% CI: −103.4%, −52.3%) (p < 0.001). After weighting, the avapritinib cohort had a significantly greater maximum reduction in serum tryptase level, with mean difference of −60.3% (95% CI: −72.8%, −47.9%) (p < 0.001). The mean time to maximum reduction after weighting was 8.8 (SD: 9.2) months in the avapritinib cohort and 8.5 (17.1) months in the BAT cohort.
Safety (BAT cohort)
A total of 250 lines of therapy, contributed by 161 BAT patients, were included in the safety analysis (Table 6). Overall, at least one AE resulting in treatment modification or discontinuation, hospitalization, or death was reported in 100 (40.0%) lines of therapy. In 1L, the most reported AEs were anemia (8.5%) followed by neutropenia (7.1%), while in 2L they were neutropenia and vomiting (5.4% each).
Analyses of OS among subgroups
The OS comparisons (weighted and unweighted) by patient subgroup are described in Table 7, Fig. 1b, and Supplementary Figs. 1–5. In the adjusted analyses, OS was longer in the avapritinib cohort than in the BAT cohort for all subgroups examined. Specifically, OS was significantly improved in the avapritinib cohorts when comparing patients who received avapritinib vs. BAT as 1L therapy (0.40 (95% CI: 0.22, 0.74); p = 0.003); avapritinib (200 mg) vs. BAT as 2L + therapy (0.37 (0.18, 0.75); p = 0.006); and avapritinib (≤200 mg) vs. BAT as 2L + therapy (0.34 (0.17, 0.69); p = 0.003). Although non-significant, a similar trend of improved OS was observed in the PATHFINDER-only analyses of avapritinib (200 mg) vs. BAT as 2L + therapy (PATHFINDER RAC-RE population: 0.47 (95% CI: 0.21, 1.09), p = 0.080; PATHFINDER safety population: 0.49 (0.20, 1.23), p = 0.127).
Discussion
This study collected data via a retrospective chart review on patients with AdvSM receiving BAT to serve as controls to patients with AdvSM who received avapritinib in the single-arm EXPLORER and PATHFINDER trials. In the absence of a randomized controlled trial, this study presents a valuable perspective on the comparative efficacy of avapritinib compared to BAT in a real-world setting.
After adjusting for differences in key prognostic factors and confounders between the two treatment cohorts, avapritinib was associated with significantly improved survival compared with BAT (HR (95% CI): 0.48 (0.29, 0.79); p = 0.004). In subgroup analyses, all subgroups of patients in the avapritinib cohort experienced a reduced risk of death compared to patients in the BAT cohort, with most comparisons statistically significant. Specifically, patients treated in 2L + with avapritinib at a dose of ≤200 mg had decreased risk of death by 66% compared to BAT. The small sample size of certain subgroups may have contributed to statistically non-significant findings. Avapritinib also offered significantly greater reduction in mast cell burden relative to BAT, with median maximum percentage reduction in serum tryptase levels of 93% compared with 37% for BAT. The results of this study, which collected patient-level data allowing for rigorous statistical analysis, further reinforce recent findings from an indirect treatment comparison of avapritinib vs. midostaurin, which compared aggregate-level data from the clinical trials and reported an adjusted HR for OS of 0.44 (95% CI: 0.25–0.76) [35].
Outcomes observed for the BAT cohort in this study are consistent with prior studies of therapies for AdvSM. In a registry-based analysis, Lübke et al. reported an OS from initiation of 1L treatment with midostaurin of 3.1 years (37 months) and OS from initiation of 1L treatment with cladribine of 1.6 years (18 months) [13]. In this study, the most commonly observed 1L therapies in the BAT cohort were midostaurin (54.2%) and cladribine (18.7%), and the mean OS from initiation of 1L treatment for the BAT cohort was 27 months, consistent with findings of Lübke et al. Jawhar et al. reported a median OS of 30 months in a study of midostaurin in 35 patients with AdvSM [16], and a larger open-label study of 116 patients by Gotlib et al. reported a median OS of 34 months [9]. The median best percentage change in serum tryptase levels in the IPTW-weighted BAT cohort was −36.9%, which is generally consistent with prior studies of midostaurin (−58% in Gotlib et al. [9] and −47% in DeAngelo et al. [15]), with differences potentially attributable to the inclusion of therapies other than midostaurin, such as cladribine, in the BAT cohort.
This study benefited from several strengths related to its methodology and employed strategies to maximize comparability between the two cohorts. These included using eligibility criteria for the BAT cohort similar to those of the EXPLORER and PATHFINDER trials, a standardized procedure for data collection across study sites, and harmonization of definitions for the outcomes and key baseline characteristics between the two treatment cohorts. Additionally, patients in the BAT cohort contributed data on multiple lines of therapy, which allowed the statistical analysis to have increased power. Finally, rigorous statistical methods such as IPTW-weighting and doubly robust estimation were used to account for the potential differences in the comprehensive list of a priori specified key adjustment covariates between the avapritinib and BAT cohorts.
The results of this study should be interpreted within the bounds of certain limitations. First, AdvSM diagnosis information collected for the BAT cohort was based on local clinician-assessed evaluation using the 2016 revision to the World Health Organization diagnostic criteria, and correct diagnosis might not have been made prior to the substantial increases in disease awareness and knowledge occurring in last decade. AdvSM diagnoses for the avapritinib cohort were based on the same criteria but confirmed by the RAC. Thus, there may have been misclassification of the clinician-assessed AdvSM diagnosis in the BAT cohort, which could result in an underestimation of the difference in OS (OS for patients with indolent SM and smoldering SM is typically longer than for patients with AdvSM) [7, 36]. However, as all participating sites are centers with expertise in the treatment of AdvSM, this concern is mitigated.
Second, AEs were described for the BAT cohort only, due to differences in definitions and data collection of AEs in EXPLORER/PATHFINDER and non-protocol clinical practice. AEs in the BAT cohort (collected retrospectively from patient charts) are expected to be underreported compared to per-protocol, prospectively collected AEs in a trial setting. In addition, trial AEs are graded per Common Terminology Criteria for Adverse Events, whereas AEs in standard clinical practice are not. This makes comparison (even non-statistical) infeasible.
Third, due to the retrospective nature of data collection for the BAT cohort, the results may have been impacted by incomplete reporting for key characteristics, such as performance status. However, a sensitivity analysis assessing the impact of missing performance status indicated that this is not expected to impact the results.
Lastly, in the primary analysis of OS, 21 (14.9%) patients from the BAT cohort went on to receive avapritinib as part of EXPLORER or PATHFINDER. These patients were included in the BAT cohort and censored at the initiation of avapritinib. Because no identifiable information was collected for real-world patients, some of these patients may have been included in the avapritinib cohort as well. These patients were censored at avapritinib initiation to ensure their time was not counted in both treatment cohorts. Additionally, given the low proportion of cross-over and resulting low impact of bias due to potential informative censoring, additional methods such as inverse probability of censoring weighting were not used for adjustment.
In conclusion, the results from this analysis show that patients with AdvSM treated with avapritinib in EXPLORER and PATHFINDER experienced significantly improved survival, longer DOT, as well as greater reductions in serum tryptase levels, compared to patients treated with BAT. Furthermore, the findings indicated a survival benefit in patients treated with avapritinib at doses of ≤200 mg across all lines compared to BAT. These data offer important insights into the superior comparative efficacy of avapritinib relative to other therapies for AdvSM.
References
Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–25.
Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–53.
Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–7.
Valent P, Akin C, Hartmann K, Alvarez-Twose I, Brockow K, Hermine O, et al. Updated diagnostic criteria and classification of mast cell disorders: a consensus proposal. HemaSphere. 2021;5:e646.
Pardanani A. Systemic mastocytosis in adults: 2021 Update on diagnosis, risk stratification and management. Am J Hematol. 2021;96:508–25.
Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues (revised 4th ed.). Lyon, France: IARC; 2017.
Lim K-H, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727–36.
Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris M-O, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013;121:1285–95.
Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2530–41.
Jawhar M, Schwaab J, Meggendorfer M, Naumann N, Horny H-P, Sotlar K, et al. The clinical and molecular diversity of mast cell leukemia with or without associated hematologic neoplasm. Haematologica. 2017;102:1035–43.
Kluin-Nelemans HC, Jawhar M, Reiter A, van Anrooij B, Gotlib J, Hartmann K, et al. Cytogenetic and molecular aberrations and worse outcome for male patients in systemic mastocytosis. Theranostics. 2021;11:292.
Reiter A, George TI, Gotlib J. New developments in diagnosis, prognostication, and treatment of advanced systemic mastocytosis. Blood. 2020;135:1365–76.
Lübke J, Schwaab J, Naumann N, Horny H-P, Weiß C, Metzgeroth G, et al. Superior efficacy of midostaurin over cladribine in advanced systemic mastocytosis: a registry-based analysis. J Clin Oncol. 2022:JCO2101849. https://doi.org/10.1200/JCO.21.01849.
Jawhar M, Schwaab J, Álvarez-Twose I, Shoumariyeh K, Naumann N, Lübke J, et al. MARS: mutation-adjusted risk score for advanced systemic mastocytosis. J Clin Oncol. 2019;37:2846–56.
DeAngelo DJ, George TI, Linder A, Langford C, Perkins C, Ma J, et al. Efficacy and safety of midostaurin in patients with advanced systemic mastocytosis: 10-year median follow-up of a phase II trial. Leukemia. 2018;32:470–8.
Jawhar M, Schwaab J, Naumann N, Horny HP, Sotlar K, Haferlach T, et al. Response and progression on midostaurin in advanced systemic mastocytosis: KIT D816V and other molecular markers. Blood. 2017;130:137–45.
Chandesris M-O, Damaj G, Canioni D, Brouzes C, Lhermitte L, Hanssens K, et al. Midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374:2605–7.
Singh A, Al‐Kali A, Begna KH, Litzow MR, Larsen JT, Sher T, et al. Midostaurin therapy for advanced systemic mastocytosis: Mayo Clinic experience in 33 consecutive cases. Am J Hematol. 2022. https://doi.org/10.1002/ajh.26498.
Alvarez-Twose I, Matito A, Morgado JM, Sánchez-Muñoz L, Jara-Acevedo M, García-Montero A, et al. Imatinib in systemic mastocytosis: a phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget. 2016;8:68950–63.
Barete S, Lortholary O, Damaj G, Hirsch I, Chandesris MO, Elie C, et al. Long-term efficacy and safety of cladribine (2-CdA) in adult patients with mastocytosis. Blood. 2015;126:1009–16.
Tefferi A, Kittur J, Farrukh F, Begna KH, Patnaik MM, Al-Kali A, et al. Cladribine therapy for advanced and indolent systemic mastocytosis: Mayo Clinic experience in 42 consecutive cases. Br J Haematol. 2022;196:975–83.
Lim KH, Pardanani A, Butterfield JH, Li CY, Tefferi A. Cytoreductive therapy in 108 adults with systemic mastocytosis: Outcome analysis and response prediction during treatment with interferon‐alpha, hydroxyurea, imatinib mesylate or 2‐chlorodeoxyadenosine. Am J Hematol. 2009;84:790–4.
Kluin-Nelemans HC, Oldhoff JM, Van Doormaal JJ, Van’t Wout JW, Verhoef G, Gerrits WB, et al. Cladribine therapy for systemic mastocytosis. Blood. 2003;102:4270–6.
Hauswirth AW, Simonitsch-Klupp I, Uffmann M, Koller E, Sperr WR, Lechner K, et al. Response to therapy with interferon alpha-2b and prednisolone in aggressive systemic mastocytosis: report of five cases and review of the literature. Leuk Res. 2004;28:249–57.
Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S, et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32:3264–74.
DeAngelo DJ, Radia DH, George TI, Robinson WA, Quiery AT, Drummond MW, et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: the phase 1 EXPLORER trial. Nat Med. 2021;27:2183–91.
Gotlib J, Reiter A, Radia DH, Deininger MW, George TI, Panse J, et al. Efficacy and safety of avapritinib in advanced systemic mastocytosis: interim analysis of the phase 2 PATHFINDER trial. Nat Med. 2021;27:2192–9.
Jawhar M, Schwaab J, Hausmann D, Clemens J, Naumann N, Henzler T, et al. Splenomegaly, elevated alkaline phosphatase and mutations in the SRSF2/ASXL1/RUNX1 gene panel are strong adverse prognostic markers in patients with systemic mastocytosis. Leukemia. 2016;30:2342–50.
Jawhar M, Schwaab J, Schnittger S, Meggendorfer M, Pfirrmann M, Sotlar K, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V+ advanced systemic mastocytosis. Leukemia. 2016;30:136–43.
Sperr WR, Kundi M, Alvarez-Twose I, van Anrooij B, Elberink JNO, Gorska A, et al. International prognostic scoring system for mastocytosis (IPSM): A retrospective cohort study. Lancet Haematol. 2019;6:e638–9.
Nelson W. Hazard plotting for incomplete failure data. J Qual Technol. 1969;1:27–52.
Aalen O. Nonparametric inference for a family of counting processes. Ann Stat. 1978;6:701–26.
Funk MJ, Westreich D, Wiesen C, Sturmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173:761–7.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79.
Pilkington H, Smith S, Roskell N, Iannazzo S. Indirect treatment comparisons of avapritinib versus midostaurin for patients with advanced systemic mastocytosis. Future Oncol. 2022. https://doi.org/10.2217/fon-2021-1509.
Pardanani A, Lim K-H, Lasho TL, Finke CM, McClure RF, Li C-Y, et al. WHO subvariants of indolent mastocytosis: clinical details and prognostic evaluation in 159 consecutive adults. Blood. 2010;115:150–1.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Acknowledgements
The “Deutsche José-Carreras Leukämie-Stiftung” (grant no. DJCLS 08R/2020) supported data collection at the University Hospital Mannheim, Germany. Morgan Johnson and Victoria Allen assisted with data collection for Dana-Farber Cancer Institute. Javier Muñoz-González, PhD, a former employee of Sofpromed Investigación Clínica, assisted with data collection for Hospital Virgen del Valle. Susanne Herndlhofer and Karoline V. Gleixner assisted with data collection for the Medical University of Vienna. Monika Ciesieslka and Rebecca Todd assisted with data collection for Guy’s and St Thomas’ NHS Foundation Trust. Assistance for data collection and statistical analysis was provided by Nisha Hazra, PhD, Sonia Cheung, Louise Yu, Estella Dong, Anamika Khanal, and Manasi Mohan, employees of Analysis Group, Inc. Shelley Batts, PhD, an employee of Analysis Group, Inc., provided drafts and editorial assistance to the authors during preparation of this manuscript.
Funding
This study was supported by Blueprint Medicines Corporation. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
AR, JG, IÁ-T, DHR, JL, JS, IAG, CP, WRS, PS, PV, and DJD were responsible for reviewing the study protocol, performing medical chart screening and abstraction, assisting with result interpretation, and providing comments on the manuscript drafts. PJB, AW, AC, SRS, and MSD were responsible for designing the study protocol, coordinating data collection, cleaning and analyzing data, interpreting results, preparing tables and figures for the manuscript, and reviewing the manuscript drafts. CN, SD, ES, and ML-G were responsible for securing funding for the study, designing the study protocol, assisting with result interpretation, and providing comments on the manuscript drafts. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
AR, JG, IÁ-T, DHR, JL, JS, IAG, CP, WRS, PS, PV, and DJD are affiliated with institutions that have received consulting fees for this study from Blueprint Medicines Corporation. CN, SD, ES, and ML-G are employees of Blueprint Medicines Corporation and hold stock/options. PJB, AW, AC, SRS, and MSD are employees of Analysis Group, Inc., which has received consulting fees from Blueprint Medicines Corporation for the conduct of this research. AR was a member of the Study Steering Committee for the global trial of midostaurin (Novartis), the RAC for studies of avapritinib in AdvSM (Blueprint Medicines Corporation), and the Study Steering Committee for the phase II trial of ripretinib in AdvSM (Deciphera Pharmaceuticals); has received research funding for the conduct of these trials; and has received honoraria and reimbursement of travel expenses from Novartis, Blueprint Medicines Corporation, and Deciphera Pharmaceuticals. JG has received consulting fees and research funding from Blueprint Medicines Corporation, Deciphera, Incyte and Kartos Therapeutics, and has served as chair of the RAC for Blueprint Medicines Corporation’ EXPLORER study, and for the PATHFINDER study, and as co-chair for the Deciphera Study Steering Committee for ripretinib in AdvSM, and chair of the Central Response Review Committee for the phase 2 study of bezuclastinib in AdvSM. IÁ-T is the principal investigator of the PATHFINDER study in Spain and has received advisory board fees from Blueprint Medicines Corporation; and has participated in educational events for Novartis. DHR has received consulting fees and research funding from Blueprint Medicines Corporation, is a member of the RACs for the EXPLORER and PATHFINDER studies, and has received honoraria from Novartis for educational events and consultancy. JS has received advisory board fees from Blueprint Medicines Corporation and Novartis. PV has received honoraria from AOP Orphan Pharmaceuticals, Blueprint Medicines Corporation, Celgene, Novartis, and Pfizer. DJD has served as a consultant for Amgen, Agios, Autolus, Blueprint Medicines Corporation, Forty-Seven, Incyte Corporation, Jazz, Novartis, Pfizer, Shire and Takeda, and received research funding from AbbVie, Blueprint Medicines Corporation, GlycoMimetics and Novartis. JL, IAG, CP, WRS, and PS have no further competing interests to report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reiter, A., Gotlib, J., Álvarez-Twose, I. et al. Efficacy of avapritinib versus best available therapy in the treatment of advanced systemic mastocytosis. Leukemia 36, 2108–2120 (2022). https://doi.org/10.1038/s41375-022-01615-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01615-z
This article is cited by
-
Allogeneic haematopoietic cell transplantation for advanced systemic mastocytosis: Best practice recommendations on behalf of the EBMT Practice Harmonisation and Guidelines Committee
Leukemia (2024)
-
Allogeneic Hematopoietic Cell Transplantation in Advanced Systemic Mastocytosis: A retrospective analysis of the DRST and GREM registries
Leukemia (2024)
-
Response and resistance to cladribine in patients with advanced systemic mastocytosis: a registry-based analysis
Annals of Hematology (2023)