Abstract
In this population-based study, we aimed to characterize and compare subgroups of therapy-related Myelodysplastic syndromes (t-MDS) and define the implications of type of previous treatment and primary disease. We combined data from MDS patients, diagnosed between 2009 and 2017 (n = 2705), in the nationwide Swedish MDS register, with several health registers. Furthermore, using matched population controls, we investigated the prevalence of antecedent malignancies in MDS patients in comparison with the general population. This first ever nationwide study on t-MDS confirms a shorter median survival for t-MDS compared to de novo MDS (15.8 months vs 31.1 months, p < 0.001). T-MDS patients previously treated with radiation only had disease characteristics with a striking resemblance to de novo-MDS, in sharp contrast to patients treated with chemotherapy who had a significantly higher risk profile. IPSS-R and the WHO classification differentiated t-MDS into different risk groups. As compared with controls, MDS patients had a six-fold increased prevalence of a previous hematological malignancy but only a 34% increased prevalence of a previous solid tumor. T-MDS patients with a previous hematological malignancy had a dismal prognosis, due both to mortality related to their primary disease and to high-risk MDS.
Similar content being viewed by others
Introduction
Myelodysplastic syndromes (MDS) diagnosed after exposure to chemotherapy and/or radiation are classified as therapy-related (t)-MDS and constitute 10–20% of all MDS [1]. Together with t-acute myeloid leukemia (AML) and t-myelodysplastic/myeloproliferative neoplasms (MDS/MPN), t-MDS is included in the entity therapy-related myeloid neoplasms (t-MN) in the 2016 World Health Organization (WHO) classification [2]. Compared to de novo-MDS, t-MDS patients have higher-risk clinical characteristics, including more cytogenetic aberrations, high-risk mutations, as well as shorter survival [1, 3, 4]. A growing numbers of cancer survivors, longer life-expectancy, and the increased use of adjuvant chemotherapy are expected to increase the future number of t-MN patients [5, 6].
The understanding of the pathogenesis of t-MN has evolved substantially during the last decade. Historically regarded as a direct consequence of cytotoxic treatment leading to DNA damage in hematopoietic stem cells, the field have shifted to a more complex understanding of the etiology of t-MN. Clonal selection of pre-existing mutant clones [7,8,9], inherited cancer predisposition [10], and the disruption of the normal bone marrow microenvironment all contribute to the development of t-MN [11].
In this study, based on the Swedish MDS register (SMDSR) and several national health registers we characterize and compare subgroups of t-MDS. Furthermore, we investigate the prevalence of malignancies preceding MDS and compare this with the general population. With our nationwide population-based data, we aim to contribute to the efforts in redefining t-MDS and its prognostic implications.
Materials and methods
Patients and data sources
All patients with a MDS diagnosis registered in the SMDSR 2009-2017 were included. The register includes detailed clinical data as described in detail elsewhere [12]. Mutational data was not available. All Swedish hospitals diagnosing MDS (n = 67) report to the register, see Supplementary Fig. 1 for a list of contributing sites and their geographical distribution. During the period under study, the completeness of the SMDSR was 97% compared to the Cancer Register to which reporting is mandatory [13]. T-MDS was defined according to the WHO 2016 classification as patients who had received any type of chemotherapy and/or radiation prior to the diagnosis of MDS.
For the purpose of the present study, we generated a dataset based on individual level record linkage between several registers with national coverage, including SMDSR, the National Patient Register, the Cancer Register, and the Cause of Death Register. These registers are described in detail in the appendix (supplemental material). The primary disease was defined as a disease diagnosed at any time before MDS diagnosis for which chemotherapy and/or radiation was given. In cases where there were multiple possible primary diseases, the most likely one was selected, based on data from all applicable registers, treatment of the particular disease and the time span between the primary disease and the diagnosis of MDS. Data on type or doses of chemotherapy and radiation were not available.
Comorbidity was estimated with Charlson comorbidity index (CCI) [14] using a recently published coding algorithm [15], including diagnoses 10 years preceding MDS diagnosis. To be able to compare comorbidity in de novo-MDS and t-MDS patients, we excluded malignancies. To compare the prevalence of malignant diseases between MDS patients and the general population, controls free from MDS and MDS/MPN at time of case diagnosis were randomly selected from the National Population Register and matched 1:5 on sex, age, and county of residence.
Causes of death were obtained up to December 31, 2018. Patients were followed for death or emigration until November 20, 2019. This study was approved by the ethics committee of Uppsala University (2014/176).
Statistical analysis
To assess the distribution of baseline patient characteristics, standard descriptive techniques were used, including chi-squared test and Wilcoxon rank sum test. Overall survival (OS) was estimated by the Kaplan–Meier method and compared using the log-rank test. Relative mortality was analyzed with Cox proportional hazards models, yielding hazard ratios (HRs) with 95% confidence intervals (CIs). Unadjusted logistic regression models were fitted to compare the likelihood of previous malignant disease between MDS patients and their matched controls, yielding odds ratios (ORs) and 95% CIs. A p-value of less than 0.05 was considered statistically significant. All analyses were performed using Stata 16 (StataCorp, TX, USA) and SPSS Statistics for Windows, Version 28 (IBM, NY, USA).
Results
Study population and patient characteristics
A total of 2705 patients were included in this study, 423 (16%) of whom were classified as t-MDS. The median follow-up for all patients was 26 months (range 0–130) and for surviving patients 56 months (range 23–130). Our dataset also included 13,509 matched controls.
Table 1 and Supplementary Table 1 show characteristics for patients with de novo-MDS, t-MDS and subgroups of t-MDS. A higher proportion of t-MDS patients were found in the high (24%) and very high (26%) Revised International Prognostic Scoring System (IPSS-R) groups compared to de novo-MDS (15% and 14%, respectively) (p < 0.001), a major contributing factor was the large number of t-MDS patients with high-risk cytogenetics (39% with poor or very poor cytogenetic risk group). The comorbidity burden was similar in patients with t-MDS and de novo-MDS, with a mean CCI of 0.84 and 0.72, respectively (p = 0.55) (data not shown).
In patients with t-MDS, 56% had been treated with chemotherapy only for their primary disease, 25% with radiation only and 19% with both (Table 1). De novo-MDS and t-MDS patients treated with radiation only had similar distribution of transfusion dependency, blast count, and cytogenetic risk group (p > 0.05 for all three variables). By contrast, t-MDS treated with chemotherapy only or chemotherapy in combination with radiation had transfusion dependency, blast count, and cytogenetic risk group with a significantly higher risk profile than de novo-MDS (p < 0.05 for all three variables). A higher proportion of t-MDS patients with a hematological malignancy as their primary disease had IPSS-R high risk or very high risk (67%) compared to solid tumor as primary disease (37%) or non-malignant primary disease (40%) (p < 0.001). Particularly, adverse cytogenetics contributed to the high scores according to IPSS-R for patients with a previous hematological disease (Supplementary Table 1).
The primary disease was a solid tumor in 176 patients (42%), among whom 95 (54%) had received radiation only (Table 2). A hematological malignancy was the primary disease in 160 patients (38%) most of these patients had received chemotherapy only (78%). A non-malignant disease was the primary disease in 63 patients (15%). Of the patient with a non-malignant disease 46 (78%) had received a prescription of methotrexate (data not shown). Information regarding primary disease was unavailable for 24 patients (6%). The median latency time between the diagnosis of the primary disease and MDS was 6.6 years and 6.5 years for solid tumors and hematological malignancy, respectively.
Overall survival
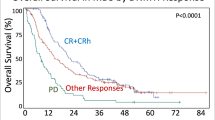
The median OS of t-MDS was 15.8 months, compared to 31.1 months for de novo-MDS (p < 0.001) (Table 1). Patients treated with chemotherapy or chemotherapy and radiation in combination had significantly shorter survival (13.3 and 9.0 months, respectively) than patients treated with radiation only (34.8 months) (p < 0.001) (Table 1, Fig. 1a).
T-MDS patients with a previous non-malignant disease and a previous solid tumor had longer OS, (26.1 and 22.3 months) compared with patients with a history of a hematological malignancy (9.0 months) (p < 0.001) (Fig. 1b, Supplementary Table 1.).
Survival according to IPSS-R and WHO classification
IPSS-R effectively discriminated different risk groups in both de novo-MDS and t-MDS (Fig. 2a, b) and in subgroup analyses of patients treated with chemotherapy only, radiation only, or a combination of chemotherapy and radiation (Fig. 2c–e). Furthermore, IPSS-R could separate risk groups for patients with solid tumor and non-malignant disease as the primary disease, but to a lesser extent for hematological disease (Fig. 2f–h). In Supplementary Fig. 2, we show that the survival in each IPSS-R risk group was similar for de novo and t-MDS in the very low, low and intermediate risk group but shorter for t-MDS in the high and very high risk group.
a OS of patients with de novo-MDS. b OS of all patients with t-MDS. c OS of patients with t-MDS treated with chemotherapy only. d OS of patients with t-MDS treated with radiation only. e OS of patients with t-MDS treated with chemotherapy in combination with radiation. f OS of patients with t-MDS with a solid tumor as a primary disease. g OS of patients with t-MDS with a hematological malignancy as a primary disease. h OS of patients with t-MDS with a non-malignant disease as a primary disease.
The OS for de novo-MDS and t-MDS according to WHO classification is illustrated in Fig. 3a, b. Since there were a limited number of patients in each WHO group, these were combined according to their median survival. MDS with isolated del(5q) and MDS-RS were combined into a good-risk group, MDS-SLD, MDS-MLD, and MDS-U were combined into an intermediate group and MDS-EB1 and MDS-EB2 into a poor-risk group. There was a difference in survival in t-MDS according to the WHO-based risk groups, good vs intermediate (p < 0.002) and intermediate vs poor (p < 0.001). The WHO classification could also discriminate different risk groups in subgroups based on type of cytotoxic treatment (Fig. 3c–e). In subgroups based on type of primary disease, the WHO classification was able to separate different risk groups in patients with solid tumors, but to a lesser extent for patients with hematological malignancies (Fig. 3f–h). The survival within the WHO-based risk groups was shorter for t-MDS compared to de novo MDS in the intermediate and poor risk group (Supplementary Fig. 3).
a OS of patients with de novo-MDS. b OS of all patients with t-MDS. c OS of patients with t-MDS treated with chemotherapy only. d OS of patients with t-MDS treated with radiation only. e OS of patients with t-MDS treated with chemotherapy in combination with radiation. f OS of patients with t-MDS with a solid tumor as a primary disease. g OS of patients with t-MDS with a hematological malignancy as a primary disease. h OS of patients with t-MDS with a non-malignant disease as a primary disease.
Uni- and multivariable analysis of all-cause mortality
In univariable analysis of the group with t-MDS, type of previous cytotoxic treatment, type of primary disease, WHO risk group, comorbidity as measured with CCI, medullary blast count, cytogenetic risk group, risk group according to IPSS-R, red blood cell and platelet-transfusion dependency were all associated with survival (Table 3). In multivariable analysis, age group, type of previous cytotoxic treatment, type of primary disease, CCI, and risk group according to IPSS-R were independently associated with survival (Fig. 4).
Causes of death
Patients with a previous hematological malignancy had their primary malignancy more often stated as their cause of death (45%) than patients with a previous solid tumor (15%) (Supplementary Table 2). Patients with previous Hodgkin’s lymphoma, chronic lymphocytic leukemia, and myeloma had their primary disease stated as underlying cause of death in their death certificate in more than 50% of cases.
Prior history of cancer in cases and controls
We observed in total 737 malignancies in 565 (20.9%) MDS patients and 2366 malignancies in 2217 (16.4%) controls. The prevalence of prior malignancies, latency between the previous malignancies and MDS and ORs are presented in Table 4. MDS patients were more likely to have had a solid tumor than controls (OR = 1.34, 95% CI: 1.21–1.49). The highest ORs for solid tumors were found for penile and testicular cancer (OR = 2.39, 95% CI: 1.12–5.08), lung, mediastinal, pleural and myocardial cancers (OR 1.92 95% CI 1.19–3.11) and head and neck cancer (OR = 1.88, 95% CI: 1.09–3.23). There was a six-fold increase for antecedent hematological malignancies in MDS patients (OR = 6.09, 95% CI: 4.87–7.61). All types of hematological malignancies were overrepresented, the most common were non-Hodgkin lymphoma, multiple myeloma and essential thrombocythemia (ET). The highest ORs for hematological malignancy were found for AML (OR = 28.5, 95% CI: 8.34–97.2), Myelofibrosis/MPN NOS (OR = 22.5, 95% CI: 4.87–104.4) and ET (OR = 18.1, 95% CI: 6.71–48.8).
Discussion
In this large nationwide population-based study on 2705 patients with MDS, including 423 patients with t-MDS, we were able to examine clinical characteristics in detail and found significant differences between subgroups of t-MDS. One of our most important findings is that t-MDS patients with previous cytotoxic treatment in the form of radiation only, have clinical characteristics and prognosis comparable to de novo-MDS. They have transfusion dependency, blast count and a cytogenetic risk profile with a striking resemblance to de novo-MDS, in sharp contrast to patients treated with chemotherapy or a combination of chemotherapy and radiation.
Previous studies, including patients from earlier time periods, indicated that patients treated with radiation only that developed MDS had a high-risk disease and short survival [3, 16, 17]. Corroborating results from other more recent studies, we found that prior cytotoxic treatment with radiation only is associated with a prognosis similar to de novo-MDS [18, 19]. One explanation for this might be that the field of radiation therapy has moved to more conformal techniques leading to a decrease in the exposure to the bone marrow, this is particularly true in lymphoma treatments and in radiation to the pelvis [20, 21].
Early studies found a very high percentage of high-risk features and a uniform poor prognosis among t-MDS patients, reporting that survival was not affected by WHO subgroups or blast percentages [3, 22]. However, as in several other more recent studies, our data shows a more heterogeneous result with a fairly large group having a normal karyotype and being low risk according to IPSS-R [1, 16, 23]. Prognostic factors such as blast percentage, transfusion dependency and particularly cytogenetic risk group, were highly predictive of OS in our cohort of t-MDS. In unadjusted analysis, age was not associated with survival but in adjusted analysis it was. We believe that the reason for this is that high risk variables such as chemotherapy and previous hematological malignancy was more common among younger patients and low risk variables such as radiation was more common in older patients. Our group and others have previously reported that IPSS-R is a powerful prognostic tool for t-MDS [1, 12, 23]. Our present study shows that this also is true for all subgroups based on type of cytotoxic treatment and type of primary disease with the exception of patients with previous hematological malignancies.
In our study, the WHO classification discriminated different risk groups in all subgroups except in patients with a previous hematological malignancy. Kuendgen et al have recently in a large collaborative study on t-MDS showed that t-MDS is as heterogeneous as de novo MDS, moreover, they showed that IPSS-R and the WHO classification effectively risk classifies t-MDS and our results are in line with this [23]. We agree in their conclusion that t-MDS should be risk stratified with available prognostic tools.
The new prognostic scoring system, IPSS-M has recently been published [24]. It incorporates mutational data with the classical IPSS-R parameters and the cohort from which it was developed included around 8% of t-MDS patients [24]. When outcomes in each risk group of IPSS-M were compared between de novo and t-MDS they were similar. In our analysis of each risk group of IPSS-R the survival was shorter for t-MDS in the higher risk groups. This indicates that IPSS-M better than IPSS-R accounts for the high risk features of t-MDS and highlights the importance of a molecular evaluation in t-MDS. It is of great value that t-MDS is included in the IPSS-M which enables a correct individual prognosis for t-MDS patients and improves the possibility to include t-MDS in future clinical trials.
Two suggestions for updated classifications of myeloid neoplasms have recently been published [25, 26]. In one of these, the group therapy-related myeloid neoplasm is omitted and first priority is given to classify the therapy-related disease according to morphologic and genetic features as for de novo disease [26]. Our results that classification and prognostication for de novo MDS is effective in t-MDS are in line with those recommendations. In the other suggestion, the authors specify that only exposure to large field radiation should be considered causing t-MN [25]. In our study, we did not have information on radiation fields. We can only speculate that many patients treated with radiation only had received smaller radiation fields. However, our results suggest that t-MDS patients treated with any kind of radiation only should be considered as de novo-MDS with regard to prognostication and treatment choices. Another proposed update is that methotrexate does no longer qualify as a cause of t-MN [25]. We lack information on type of given chemotherapy for the primary condition, but we had access to data from the prescription register. We can conclude that patients with t-MDS with a non-malignant disease, of whom a majority had been treated with methotrexate, had a prognosis similar to de novo-MDS. This finding supports omitting methotrexate as a cause of t-MDS.
Our results show that mortality from the primary disease is substantial, with the highest mortality observed in patients with a previous hematological malignancy. This group of patients have a dismal prognosis with only nine months median OS. Besides a high risk of dying from their primary disease, most of them have high-risk MDS. IPSS-R and WHO classification were less effective in discriminating risk groups in t-MDS with a previous hematological disease. Based on our findings we can conclude that both primary disease and type of cytotoxic treatment strongly influence survival and that these additional variables should be considered in the prognostication of t-MDS.
One part of our study was conducted with a case-control approach comparing the history of prior malignancies between MDS patients and controls. Malignancies that usually include chemotherapy in their treatment such as colon, gynecological cancers and head and neck cancers had higher ORs, while malignancies usually treated with radiation only, such as prostate cancer, rectal cancer and thyroid cancers, had a similar prevalence in MDS patients and controls. However, firm conclusions are hard to draw due to the lack of information on the type of treatment given in the controls. The very high ORs observed for hematological malignancies are striking, particularly for myeloid malignancies. There might be a few cases with AML and MPNs where diagnostic difficulties and overlaps with MDS exist, but the long latency suggests that they represent separate previous conditions. Shared pathophysiological mechanisms and risk factors such as clonal hematopoiesis exists between the MPNs, AML, and MDS and can represent an explanation in addition to the therapy for the primary disease [27, 28]. Other non-myeloid hematological malignancies such as lymphoma and myeloma were also highly overrepresented in MDS patients. These malignancies are often treated with high doses of chemotherapy, which might lead to higher risk of t-MDS than other malignancies [29,30,31]. Autologous hematopoietic cell transplantation is used to treat both lymphomas and myeloma and is known to be associated with the development of t-MDS [32].
Strengths of our study includes its large size, based on virtually complete nationwide data from several high-quality population-based health registers and the availability of matched population controls. Limitations included the absence of information on details of the prior malignancy, including treatment such as specific types or doses of chemotherapy, dose of radiation or radiation field.
To the best of our knowledge, this is the first nationwide epidemiological study on t-MDS including analyses of different subgroups based on primary disease and type of therapy. Our findings show that primary disease and type of cytotoxic treatment strongly influence clinical characteristics and prognosis. T-MDS patients with previous cytotoxic treatment in the form of radiation only have clinical characteristics and prognosis comparable to de novo-MDS and should be viewed as de novo-MDS with regard to prognostication and treatment. We conclude that genetic and morphologic classification as well as risk stratification intended for de novo-MDS is meaningful in t-MDS and we suggest that t-MDS should be classified the same way de novo MDS is but with recognition that type of prior disease and cytotoxic treatment affects the prognosis. This is of particular importance in the group with a previous hematological malignancy where the outcomes are dismal. Taken together, our findings provide further evidence of the importance of an individualized approach in the management of t-MDS.
Data availability
The datasets generated and/or analysed in the current study are not publicly available due to privacy concerns and limitations from the ethical review board but may be available from the corresponding author upon request.
References
Zeidan AM, Al Ali N, Barnard J, Padron E, Lancet JE, Sekeres MA, et al. Comparison of clinical outcomes and prognostic utility of risk stratification tools in patients with therapy-related vs de novo myelodysplastic syndromes: a report on behalf of the MDS Clinical Research Consortium. Leukemia. 2017;31:1391–7.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102:43–52.
Chung J, Sallman DA, Padron E. TP53 and therapy-related myeloid neoplasms. Best Pract Res Clin Haematol. 2019;32:98–103.
Rowland JH, Bellizzi KM. Cancer survivorship issues: life after treatment and implications for an aging population. J Clin Oncol: Off J Am Soc Clin Oncol. 2014;32:2662–8.
Morton LM, Dores GM, Schonfeld SJ, Linet MS, Sigel BS, Lam CJK, et al. Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern era. JAMA Oncol. 2019;5:318–25.
Kuzmanovic T, Patel BJ, Sanikommu SR, Nagata Y, Awada H, Kerr CM, et al. Genomics of therapy-related myeloid neoplasms. Haematologica. 2020;105:e98–e101.
Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518:552–5.
Takahashi K, Wang F, Kantarjian H, Doss D, Khanna K, Thompson E, et al. Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: a case-control study. Lancet Oncol. 2017;18:100–11.
Churpek JE, Marquez R, Neistadt B, Claussen K, Lee MK, Churpek MM, et al. Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer. 2016;122:304–11.
McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17:513–27.
Moreno Berggren D, Folkvaljon Y, Engvall M, Sundberg J, Lambe M, Antunovic P, et al. Prognostic scoring systems for myelodysplastic syndromes (MDS) in a population-based setting: a report from the Swedish MDS register. Br J Haematol. 2018;181:614–27.
Ejerblad E. Myelodysplastiskt syndrom (MDS) Rapport för diagnosår 2009-2017. Regionalt cancercentrum, Uppsala Örebro; 2018 2018/12/21.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Ludvigsson JF, Appelros P, Askling J, Byberg L, Carrero JJ, Ekstrom AM, et al. Adaptation of the Charlson comorbidity index for register-based research in Sweden. Clin Epidemiol. 2021;13:21–41.
Quintas-Cardama A, Daver N, Kim H, Dinardo C, Jabbour E, Kadia T, et al. A prognostic model of therapy-related myelodysplastic syndrome for predicting survival and transformation to acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2014;14:401–10.
Patel AA, Rojek AE, Drazer MW, Weiner H, Godley LA, Le Beau MM, et al. Therapy-related myeloid neoplasms in 109 patients after radiation monotherapy. Blood Adv. 2021;5:4140–8.
Ok CY, Hasserjian RP, Fox PS, Stingo F, Zuo Z, Young KH, et al. Application of the international prognostic scoring system-revised in therapy-related myelodysplastic syndromes and oligoblastic acute myeloid leukemia. Leukemia. 2014;28:185–9.
Nardi V, Winkfield KM, Ok CY, Niemierko A, Kluk MJ, Attar EC, et al. Acute myeloid leukemia and myelodysplastic syndromes after radiation therapy are similar to de novo disease and differ from other therapy-related myeloid neoplasms. J Clin Oncol: Off J Am Soc Clin Oncol. 2012;30:2340–7.
Engert A, Schiller P, Josting A, Herrmann R, Koch P, Sieber M, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol: Off J Am Soc Clin Oncol. 2003;21:3601–8.
Fiorino C, Valdagni R, Rancati T, Sanguineti G. Dose-volume effects for normal tissues in external radiotherapy: pelvis. Radiother Oncol. 2009;93:153–67.
Singh ZN, Huo D, Anastasi J, Smith SM, Karrison T, Le Beau MM, et al. Therapy-related myelodysplastic syndrome: morphologic subclassification may not be clinically relevant. Am J Clin Pathol. 2007;127:197–205.
Kuendgen A, Nomdedeu M, Tuechler H, Garcia-Manero G, Komrokji RS, Sekeres MA, et al. Therapy-related myelodysplastic syndromes deserve specific diagnostic sub-classification and risk-stratification-an approach to classification of patients with t-MDS. Leukemia. 2021;35:835–49.
Bernard E, Tuechler H, Greenberg PL, Hasserjian RP, Ossa JEA, Nannya Y, et al. Molecular international prognostic scoring system for myelodysplastic syndromes. NEJM Evid. 2022;1:EVIDoa2200008.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703–19.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemia: integrating morphological, clinical, and genomic data. Blood. 2022;140:1200–28.
Nangalia J, Green AR. Myeloproliferative neoplasms: from origins to outcomes. Blood. 2017;130:2475–83.
Constantinescu SN, Vainchenker W, Levy G, Papadopoulos N. Functional consequences of mutations in myeloproliferative neoplasms. Hemasphere. 2021;5:e578.
Jonsdottir G, Bjorkholm M, Turesson I, Hultcrantz M, Diamond B, Porwit A, et al. Cumulative exposure to melphalan chemotherapy and subsequent risk of developing acute myeloid leukemia and myelodysplastic syndromes in patients with multiple myeloma. Eur J Haematol. 2021;107:275–82.
Benjamini O, Jain P, Trinh L, Qiao W, Strom SS, Lerner S, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma. 2015;56:1643–50.
Joelsson J, Wasterlid T, Rosenquist R, Jakobsen LH, El-Galaly TC, Smedby KE, et al. Incidence and time trends of second primary malignancies after non-Hodgkin lymphoma: a Swedish population-based study. Blood Adv. 2022;6:2657–66.
Radivoyevitch T, Dean RM, Shaw BE, Brazauskas R, Tecca HR, Molenaar RJ, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome after autotransplants for lymphomas and plasma cell myeloma. Leuk Res. 2018;74:130–6.
Acknowledgements
The authors wish to thank all Swedish hematologists and nurses who have reported patients to the SMDSR. The authors also appreciate the work of data managers at the respective RCC.
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
DMB designed the study, analyzed the data and wrote the paper. HG, PWH, EHL, LN, and BR are local coordinators of the SMDSR; they provided data. CW provided statistical support. ML, SL, EHL, and MJ helped designing the study. EE provided data, designed the study and analyzed the data. All authors critically reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moreno Berggren, D., Garelius, H., Willner Hjelm, P. et al. Therapy-related MDS dissected based on primary disease and treatment—a nationwide perspective. Leukemia 37, 1103–1112 (2023). https://doi.org/10.1038/s41375-023-01864-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-023-01864-6