Abstract
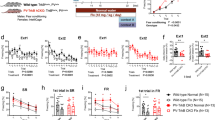
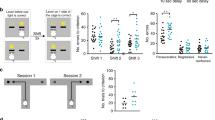
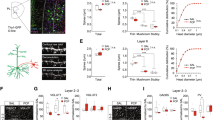
Synaptic deficit-induced excitation and inhibition (E/I) imbalance have been implicated in the pathogenesis of schizophrenia. Using in vivo two-photon microscopy, we examined the dynamic plasticity of dendritic spines of pyramidal neurons (PNs) and “en passant” axonal bouton of parvalbumin-expressing interneurons (PVINs) in the frontal association (FrA) cortex in two adolescent mouse models with schizophrenia-like behaviors. Simultaneous imaging of PN dendritic spines and PV axonal boutons showed that repeated exposure to N-methyl-D-aspartate receptor (NMDAR) antagonist MK801 during adolescence disrupted the normal developmental balance of excitatory and inhibitory synaptic structures. This MK801-induced structural E/I imbalance significantly correlated with animal recognition memory deficits and could be ameliorated by environmental enrichment (EE). In addition, selective chemogenetic activation of PVINs in the FrA mimicked the effects of EE on both synaptic plasticity and animal behavior, while selective inhibition of PVIN abolished EE’s beneficial effects. Electrophysiological recordings showed that chronic MK801 treatment significantly suppressed the frequency of mEPSC/mIPSC ratio of layer (L) 2/3 PNs and significantly reduced the resting membrane potential of PVINs, the latter was rescued by selective activation of PVINs. Such manipulations of PVINs also showed similar effects in PV-Cre; ErbB4fl/fl animal model with schizophrenia-like behaviors. EE or selective activation of PVINs in the FrA restored behavioral deficits and structural E/I imbalance in adolescent PV-Cre; ErbB4fl/fl mice, while selective inhibition of PVINs abolished EE’s beneficial effects. Our findings suggest that the PVIN activity in the FrA plays a crucial role in regulating excitatory and inhibitory synaptic structural dynamics and animal behaviors, which may provide a potential therapeutic target for schizophrenia treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Andreasen N. Symptoms, signs, and diagnosis of schizophrenia. Lancet. 1995;346:477–81.
Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull. 2011;37:504–13.
Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–57.
Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107.
Marin O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat Med. 2016;22:1229–38.
Mukherjee A, Carvalho F, Eliez S, Caroni P. Long-lasting rescue of network and cognitive dysfunction in a genetic schizophrenia model. Cell. 2019;178:1387–402 e1314.
Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol. 2012;213:267–95.
Malhotra MDA. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–50.
Lahti A. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19.
Snyder MA, Gao WJ. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci. 2013;7:31.
Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, et al. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharm Ther. 2010;128:419–32.
Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci. 2008;1:6.
Ruddy RM, Chen Y, Milenkovic M, Ramsey AJ. Differential effects of NMDA receptor antagonism on spine density. Synapse. 2015;69:52–56.
Ramsey AJ, Milenkovic M, Oliveira AF, Escobedo-Lozoya Y, Seshadri S, Salahpour A, et al. Impaired NMDA receptor transmission alters striatal synapses and DISC1 protein in an age-dependent manner. Proc Natl Acad Sci USA. 2011;108:5795–800.
Xi D, Zhang W, Wang HX, Stradtman GG, Gao WJ. Dizocilpine (MK-801) induces distinct changes of N-methyl-D-aspartic acid receptor subunits in parvalbumin-containing interneurons in young adult rat prefrontal cortex. Int J Neuropsychopharmacol. 2009;12:1395–408.
Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–83.
Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res. 2015;167:98–107.
Ferguson BR, Gao WJ. PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits. 2018;12:37.
Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345:1255263.
Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–24.
Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–14.
Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–88.
Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67.
Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–40.
Lewis DA. Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol. 2014;26:22–26.
Lewis DA, Fish KN, Arion D, Gonzalez-Burgos G. Perisomatic inhibition and cortical circuit dysfunction in schizophrenia. Curr Opin Neurobiol. 2011;21:866–72.
Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology. 2003;28:265–75.
Morrow BA, Elsworth JD, Roth RH. Repeated phencyclidine in monkeys results in loss of parvalbumin-containing axo-axonic projections in the prefrontal cortex. Psychopharmacology. 2007;192:283–90.
Nicodemus KK, Luna A, Vakkalanka R, Goldberg T, Egan M, Straub RE, et al. Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. Mol Psychiatry. 2006;11:1062–5.
Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–64.
Del Pino I, Garcia-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martinez de Lagran M, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–68.
Chen YJ, Zhang M, Yin DM, Wen L, Ting A, Wang P, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci USA. 2010;107:21818–23.
Lu Y, Sun XD, Hou FQ, Bi LL, Yin DM, Liu F, et al. Maintenance of GABAergic activity by neuregulin 1-ErbB4 in amygdala for fear memory. Neuron. 2014;84:835–46.
Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci USA. 2010;107:1211–6.
Jung CK, Herms J. Structural dynamics of dendritic spines are influenced by an environmental enrichment: an in vivo imaging study. Cereb Cortex. 2014;24:377–84.
Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709.
Kempermann G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat Rev Neurosci. 2019;20:235–45.
Urakawa S, Takamoto K, Hori E, Sakai N, Ono T, Nishijo H. Rearing in enriched environment increases parvalbumin-positive small neurons in the amygdala and decreases anxiety-like behavior of male rats. BMC Neurosci. 2013;14:13.
Murueta-Goyena A, Ortuzar N, Gargiulo PA, Lafuente JV, Bengoetxea H. Short-term exposure to enriched environment in adult rats restores MK-801-induced cognitive deficits and gabaergic interneuron immunoreactivity loss. Mol Neurobiol. 2018;55:26–41.
Koseki T, Mouri A, Mamiya T, Aoyama Y, Toriumi K, Suzuki S, et al. Exposure to enriched environments during adolescence prevents abnormal behaviours associated with histone deacetylation in phencyclidine-treated mice. Int J Neuropsychopharmacol. 2012;15:1489–501.
Burrows EL, McOmish CE, Buret LS, Van den Buuse M, Hannan AJ. Environmental enrichment ameliorates behavioral impairments modeling schizophrenia in mice lacking metabotropic glutamate receptor 5. Neuropsychopharmacology. 2015;40:1947–56.
Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97.
Chen SX, Kim AN, Peters AJ, Komiyama T. Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat Neurosci. 2015;18:1109–15.
Lai CSW, Adler A, Gan WB. Fear extinction reverses dendritic spine formation induced by fear conditioning in the mouse auditory cortex. Proc Natl Acad Sci USA. 2018;115:9306–11.
Ng LHL, Huang Y, Han L, Chang RC, Chan YS, Lai CSW. Ketamine and selective activation of parvalbumin interneurons inhibit stress-induced dendritic spine elimination. Transl Psychiatry. 2018;8:272.
Cichon J, Gan WB. Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature. 2015;520:180–5.
Saito T. In vivo electroporation in the embryonic mouse central nervous system. Nat Protoc. 2006;1:1552–8.
Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–4.
Grillo FW, Song S, Teles-Grilo Ruivo LM, Huang L, Gao G, Knott GW, et al. Increased axonal bouton dynamics in the aging mouse cortex. Proc Natl Acad Sci USA. 2013;110:E1514–1523.
Lai CSW, Franke TF, Gan W-B. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91.
Nozari M, Shabani M, Hadadi M, Atapour N. Enriched environment prevents cognitive and motor deficits associated with postnatal MK-801 treatment. Psychopharmacology. 2014;231:4361–70.
Nozari M, Shabani M, Farhangi AM, Mazhari S, Atapour N. Sex-specific restoration of MK-801-induced sensorimotor gating deficit by environmental enrichment. Neuroscience. 2015;299:28–34.
Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91.
Nakayama D, Baraki Z, Onoue K, Ikegaya Y, Matsuki N, Nomura H. Frontal association cortex is engaged in stimulus integration during associative learning. Curr Biol. 2015;25:117–23.
Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–93.
Chen CC, Lu J, Yang R, Ding JB, Zuo Y. Selective activation of parvalbumin interneurons prevents stress-induced synapse loss and perceptual defects. Mol Psychiatry. 2018;23:1614–25.
Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–7.
Ilg AK, Enkel T, Bartsch D, Bahner F. Behavioral effects of acute systemic low-dose clozapine in wild-type rats: implications for the use of DREADDs in behavioral neuroscience. Front Behav Neurosci. 2018;12:173.
Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013;16:210–8.
Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, Ecker AS, et al. Principles of connectivity among morphologically defined cell types in adult neocortex. Science. 2015;350:aac9462.
Yin DM, Sun XD, Bean JC, Lin TW, Sathyamurthy A, Xiong WC, et al. Regulation of spine formation by ErbB4 in PV-positive interneurons. J Neurosci. 2013;33:19295–303.
Takuma K, Ago Y, Matsuda T. Preventive effects of an enriched environment on rodent psychiatric disorder models. J Pharm Sci. 2011;117:71–76.
Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:e623.
Laviola G, Macrı̀ S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31.
Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–5.
Pietropaolo S, Crusio WE. Strain-dependent changes in acoustic startle response and its plasticity across adolescence in mice. Behav Genet. 2009;39:623–31.
Spanos M, Besheer J, Hodge CW. Increased sensitivity to alcohol induced changes in ERK Map kinase phosphorylation and memory disruption in adolescent as compared to adult C57BL/6J mice. Behav Brain Res. 2012;230:158–66.
Tuan LH, Lee LJ. Microglia-mediated synaptic pruning is impaired in sleep-deprived adolescent mice. Neurobiol Dis. 2019;130:104517.
Deslauriers J, Larouche A, Sarret P, Grignon S. Combination of prenatal immune challenge and restraint stress affects prepulse inhibition and dopaminergic/GABAergic markers. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:156–64.
Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87:95–102.
Shamir A, Kwon OB, Karavanova I, Vullhorst D, Leiva-Salcedo E, Janssen MJ, et al. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci. 2012;32:2988–97.
Carrion RE, Demmin D, Auther AM, McLaughlin D, Olsen R, Lencz T, et al. Duration of attenuated positive and negative symptoms in individuals at clinical high risk: associations with risk of conversion to psychosis and functional outcome. J Psychiatr Res. 2016;81:95–101.
Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–34.
Lewis R. Should cognitive deficit be a diagnostic criterion for schizophrenia? J Psychiatry Neurosci. 2004;29:102–13.
Sponheim SR, Jung RE, Seidman LJ, Mesholam-Gately RI, Manoach DS, O’Leary DS, et al. Cognitive deficits in recent-onset and chronic schizophrenia. J Psychiatr Res. 2010;44:421–8.
Murueta-Goyena A, Morera-Herreras T, Miguelez C, Gutierrez-Ceballos A, Ugedo L, Lafuente JV, et al. Effects of adult enriched environment on cognition, hippocampal-prefrontal plasticity and NMDAR subunit expression in MK-801-induced schizophrenia model. Eur Neuropsychopharmacol. 2019;29:590–600.
Bator E, Latusz J, Wedzony K, Mackowiak M. Adolescent environmental enrichment prevents the emergence of schizophrenia-like abnormalities in a neurodevelopmental model of schizophrenia. Eur Neuropsychopharmacol. 2018;28:97–108.
Madhavadas S, Subramanian S, Kutty BM. Environmental enrichment improved cognitive deficits more in peri-adolescent than in adult rats after postnatal monosodium glutamate treatment. Physiol Int. 2017;104:271–90.
Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2008;34:374–89.
Tjia M, Yu X, Jammu LS, Lu J, Zuo Y. Pyramidal neurons in different cortical layers exhibit distinct dynamics and plasticity of apical dendritic spines. Front Neural Circuits. 2017;11:43.
Hubener M, Bonhoeffer T. Neuronal plasticity: beyond the critical period. Cell. 2014;159:727–37.
Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–2.
Kawaguchi Y. Pyramidal cell subtypes and their synaptic connections in layer 5 of rat frontal cortex. Cereb Cortex. 2017;27:5755–71.
Volk DW, Lewis DA. Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci. 2010;4:485–508.
Lewis DA. Cortical circuit dysfunction and cognitive deficits in schizophrenia-implications for preemptive interventions. Eur J Neurosci. 2012;35:1871–8.
Fish KN, Hoftman GD, Sheikh W, Kitchens M, Lewis DA. Parvalbumin-containing chandelier and basket cell boutons have distinctive modes of maturation in monkey prefrontal cortex. J Neurosci. 2013;33:8352–8.
Ferguson BR, Gao WJ. Thalamic control of cognition and social behavior via regulation of gamma-aminobutyric acidergic signaling and excitation/inhibition balance in the medial prefrontal cortex. Biol Psychiatry. 2018;83:657–69.
Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015;15:146–67.
Kennedy MB. Synaptic signaling in learning and memory. Cold Spring Harb Perspect Biol. 2013;8:a016824.
Delevich K, Jaaro-Peled H, Penzo M, Sawa A, Li B. Parvalbumin interneuron dysfunction in a thalamo-prefrontal cortical circuit in disc1 locus impairment mice. eNeuro. 2020;7:ENEURO.0496-19.2020.
Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8.
Fan Z, Hu H. Medial prefrontal cortex excitation/inhibition balance and schizophrenia-like behaviors regulated by thalamic inputs to interneurons. Biol Psychiatry. 2018;83:630–1.
Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–6.
Parnaudeau S, Bolkan SS, Kellendonk C. The mediodorsal thalamus: an essential partner of the prefrontal cortex for cognition. Biol Psychiatry. 2018;83:648–56.
Delevich K, Tucciarone J, Huang ZJ, Li B. The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J Neurosci. 2015;35:5743–53.
Alvarez DD, Giacomini D, Yang SM, Trinchero MF, Temprana SG, Buttner KA, et al. A disynaptic feedback network activated by experience promotes the integration of new granule cells. Science. 2016;354:459–65.
Acknowledgements
The authors would like to thank the HKU Faculty Core Facility for their technical support on imaging experiments and the HKU Laboratory Animal Unit for their support on animal husbandry. The authors would like to thank Leonard W. Cheung for his support on mouse genotyping. The authors would like to thank Christ C. Wong for his support on MATLAB-based programs. This work was supported by the Hong Kong Research Grants Council (RGC/ECS 27103715 and RGC/GRF 17128816 to CSWL, RGC/ECS 21103818 and RGC/GRF 11104320 to CGL), the National Natural Science Foundation of China (NSFC/General Program 31571031 to CSWL), Shenzhen General Basic Research Program (JCYJ20190808182203591 to CGL), and the Health and Medical Research Fund (HMRF 03143096 to CSWL).
Author information
Authors and Affiliations
Contributions
YH and CSWL designed the experiments. YH performed behavioral tests, DREADDs viral infections, in vivo imaging experiments, and analyzed the data. HJ and CGL carried out the in vitro electrophysiological experiments and analyzed the data. QZ performed immunostaining experiments and analyzed PV somatic boutons imaging data. AHKF performed plasmid constructs cloning and in utero electroporation experiments. XL performed stereotaxic retrograde viral injections. YH and CSWL wrote and edited the manuscript with comments from all of the other authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Huang, Y., Jiang, H., Zheng, Q. et al. Environmental enrichment or selective activation of parvalbumin-expressing interneurons ameliorates synaptic and behavioral deficits in animal models with schizophrenia-like behaviors during adolescence. Mol Psychiatry 26, 2533–2552 (2021). https://doi.org/10.1038/s41380-020-01005-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-01005-w
This article is cited by
-
Effects of environmental enrichment and sexual dimorphism on the expression of cerebellar receptors in C57BL/6 and BTBR + Itpr3tf/J mice
BMC Research Notes (2022)
-
Chronic hM3Dq-DREADD-mediated chemogenetic activation of parvalbumin-positive inhibitory interneurons in postnatal life alters anxiety and despair-like behavior in adulthood in a task- and sex-dependent manner
Journal of Biosciences (2022)