Abstract
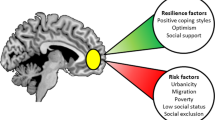
Expert opinion remains divided concerning the impact of putative risk factors on vulnerability to depression and other stress-related disorders. A large body of literature has investigated gene by environment interactions, particularly between the serotonin transporter polymorphism (5-HTTLPR) and negative environments, on the risk for depression. However, fewer studies have simultaneously investigated the outcomes in both negative and positive environments, which could explain some of the inconclusive findings. This is embodied by the concept of differential susceptibility, i.e., the idea that certain common gene polymorphisms, prenatal factors, and traits make some individuals not only disproportionately more susceptible and responsive to negative, vulnerability-promoting environments, but also more sensitive and responsive to positive, resilience-enhancing environmental conditions. Although this concept from the field of developmental psychology is well accepted and supported by behavioral findings, it is striking that its implementation in neuropsychiatric research is limited and that underlying neural mechanisms are virtually unknown. Based on neuroimaging studies that examined how factors mediating differential susceptibility affect brain function, we posit that environmental sensitivity manifests in increased salience network activity, increased salience and default mode network connectivity, and increased salience and central executive network connectivity. These changes in network function may bring about automatic exogenous attention for positive and negative stimuli and flexible attentional set-shifting. We conclude with a call to action; unraveling the neural mechanisms through which differential susceptibility factors mediate vulnerability and resilience may lead us to personalized preventive interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31.
Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–27.
Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–54.
Bleys D, Luyten P, Soenens B, Claes S. Gene-environment interactions between stress and 5-HTTLPR in depression: a meta-analytic update. J Affect Disord. 2018;226:339–45.
Sharpley CF, Palanisamy SK, Glyde NS, Dillingham PW, Agnew LL. An update on the interaction between the serotonin transporter promoter variant (5-HTTLPR), stress and depression, plus an exploration of non-confirming findings. Behav Brain Res. 2014;273:89–105.
Culverhouse RC, Saccone NL, Horton AC, Ma Y, Anstey KJ, Banaschewski T, et al. Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Mol Psychiatry 2017;23:133–42.
Risch N, Herrell R, Lehner T, Liang K-Y, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–71.
Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–9.
Zammit S, Owen MJ. Stressful life events, 5-HTT genotype and risk of depression. Br J Psychiatry. 2006;188:199–201.
Monroe SM, Reid MW. Gene-environment interactions in depression research: genetic polymorphisms and life-stress polyprocedures. Psychol Sci. 2008;19:947–56.
Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. BiolPsychiatry. 2011;69:513–9.
Fox E, Beevers CG. Differential sensitivity to the environment: contribution of cognitive biases and genes to psychological wellbeing. Mol Psychiatry. 2016;21:1657–62.
Fox E, Zougkou K, Ridgewell A, Garner K. The serotonin transporter gene alters sensitivity to attention bias modification: evidence for a plasticity gene. Biol Psychiatry. 2011;70:1049–54.
Beevers CG, Marti CN, Lee HJ, Stote DL, Ferrell RE, Hariri AR, et al. Associations between serotonin transporter gene promoter region (5-HTTLPR) polymorphism and gaze bias for emotional information. J Abnorm Psychol. 2011;120:187–97.
Beevers CG, Ellis AJ, Wells TT, McGeary JE. Serotonin transporter gene promoter region polymorphism and selective processing of emotional images. Biol Psychol. 2010;83:260–5.
Dainer-Best J, Disner SG, McGeary JE, Hamilton BJ, Beevers CG. Negative self-referential processing is associated with genetic variation in the serotonin transporter-linked polymorphic region (5-HTTLPR): evidence from two independent studies. PLoS ONE. 2018;13:e0198950.
van Roekel E, Verhagen M, Engels R, Kuppens P. Variation in the serotonin transporter polymorphism (5-HTTLPR) and inertia of negative and positive emotions in daily life. Emotion. 2018;18:229–36.
Weeland J, Slagt M, Brummelman E, Matthys W, de Castro BO, Overbeek G. 5-HTTLPR expression outside the skin: an experimental test of the emotional reactivity hypothesis in children. PLoS ONE. 2015;10:e0141474.
Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA. 2004;101:17316–21.
Mitchell C, Notterman D, Brooks-Gunn J, Hobcraft J, Garfinkel I, Jaeger K, et al. Role of mother’s genes and environment in postpartum depression. Proc Natl Acad Sci USA. 2011;108:8189–93.
Li JJ, Berk MS, Lee SS. Differential susceptibility in longitudinal models of gene-environment interaction for adolescent depression. Dev Psychopathol. 2013;25:991–1003.
Starr LR, Hammen C, Brennan PA, Najman JM. Relational security moderates the effect of serotonin transporter gene polymorphism (5-HTTLPR) on stress generation and depression among adolescents. J Abnorm Child Psychol. 2013;41:379–88.
Carola V, Gross C. Mouse models of the 5-HTTLPR x stress risk factor for depression. Curr Top Behav Neurosci. 2012;12:59–72.
Nonkes LJ, van de Vondervoort II, Homberg JR. The attribution of incentive salience to an appetitive conditioned cue is not affected by knockout of the serotonin transporter in rats. Behav Brain Res. 2014;259:268–73.
Nonkes LJ, de Pooter M, Homberg JR. Behavioural therapy based on distraction alleviates impaired fear extinction in male serotonin transporter knockout rats. J Psychiatry Neurosci. 2012;37:224–30.
Verheij MMM, Contet C, Karel P, Latour J, van der Doelen RHA, Geenen B, et al. Median and dorsal raphe serotonergic neurons control moderate versus compulsive cocaine intake. Biol Psychiatry 2017;83:1024–35.
Nonkes LJ, Maes JH, Homberg JR. Improved cognitive flexibility in serotonin transporter knockout rats is unchanged following chronic cocaine self-administration. Addict Biol. 2013;18:434–40.
Homberg JR, De Boer SF, Raaso HS, Olivier JDA, Verheul M, Ronken E, et al. Adaptations in pre- and postsynaptic 5-HT1A receptor function and cocaine supersensitivity in serotonin transporter knockout rats. Psychopharmacology. 2008;200:367–80.
Kastner N, Richter SH, Lesch KP, Schreiber RS, Kaiser S, Sachser N. Benefits of a “vulnerability gene”? A study in serotonin transporter knockout mice. Behav Brain Res. 2015;283:116–20.
Rogers J, Li S, Lanfumey L, Hannan AJ, Renoir T. Environmental enrichment reduces innate anxiety with no effect on depression-like behaviour in mice lacking the serotonin transporter. Behav Brain Res. 2017;332:355–61.
Roversi K, Buizza C, Brivio P, Calabrese F, Verheij MMM, Antoniazza CTD, et al. Neonatal tactile stimulation alters behaviors in heterozygous serotonin transporter male rats: role of the amygdala. Front Behav Neurosci. 2020;14:142.
Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Dev Psychopathol. 2008;20:745–74.
Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull. 1991;110:406–25.
Belsky J. Variation in susceptibility to rearing influence: an evolutionary argument. Psychol Inq. 1997;8:182–6.
Slagt M, Dubas JS, Ellis BJ, van Aken MAG, Dekovic M. Linking emotional reactivity “for better and for worse” to differential susceptibility to parenting among kindergartners. Dev Psychopathol. 2019;31:741–58.
Pluess M, Belsky J. Prenatal programming of postnatal plasticity? Dev Psychopathol. 2011;23:29–38.
van Ijzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Transl Psychiatry. 2012;2:e147.
Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: new evidence and a meta-analysis. Dev Psychopathol. 2011;23:39–52.
Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–54.
Mills-Koonce WR, Propper CB, Gariepy JL, Blair C, Garrett-Peters P, Cox MJ. Bidirectional genetic and environmental influences on mother and child behavior: the family system as the unit of analyses. Dev Psychopathol. 2007;19:1073–87.
Zhang L, Li Z, Chen J, Li X, Zhang J, Belsky J. The BDNF Val66Met polymorphism interacts with maternal parenting influencing adolescent depressive symptoms: evidence of differential susceptibility model. J Youth Adolesc. 2016;45:471–83.
Rich ME, deCardenas EJ, Lee HJ, Caldwell HK. Impairments in the initiation of maternal behavior in oxytocin receptor knockout mice. PLoS ONE. 2014;9:e98839.
Flasbeck V, Moser D, Kumsta R, Brune M. The OXTR single-nucleotide polymorphism rs53576 moderates the impact of childhood maltreatment on empathy for social pain in female participants: evidence for differential susceptibility. Front Psychiatry. 2018;9:359.
DiLalla LF, Bersted K, John SG. Peer victimization and DRD4 genotype influence problem behaviors in young children. J Youth Adolesc. 2015;44:1478–93.
Green CG, Babineau V, Jolicoeur-Martineau A, Bouvette-Turcot AA, Minde K, Sassi R, et al. Prenatal maternal depression and child serotonin transporter linked polymorphic region (5-HTTLPR) and dopamine receptor D4 (DRD4) genotype predict negative emotionality from 3 to 36 months. Dev Psychopathol. 2017;29:901–17.
Schmidt LA, Fox NA, Hamer DH. Evidence for a gene-gene interaction in predicting children’s behavior problems: association of serotonin transporter short and dopamine receptor D4 long genotypes with internalizing and externalizing behaviors in typically developing 7-year-olds. Dev Psychopathol. 2007;19:1105–16.
Drury SS, Gleason MM, Theall KP, Smyke AT, Nelson CA, Fox NA, et al. Genetic sensitivity to the caregiving context: the influence of 5httlpr and BDNF val66met on indiscriminate social behavior. Physiol Behav. 2012;106:728–35.
Ioannidis JP, Trikalinos TA, Khoury MJ. Implications of small effect sizes of individual genetic variants on the design and interpretation of genetic association studies of complex diseases. Am J Epidemiol. 2006;164:609–14.
Keers R, Coleman JR, Lester KJ, Roberts S, Breen G, Thastum M, et al. A genome-wide test of the differential susceptibility hypothesis reveals a genetic predictor of differential response to psychological treatments for child anxiety disorders. Psychother Psychosom. 2016;85:146–58.
Poehlmann J, Schwichtenberg AJ, Shlafer RJ, Hahn E, Bianchi JP, Warner R. Emerging self-regulation in toddlers born preterm or low birth weight: differential susceptibility to parenting? Dev Psychopathol. 2011;23:177–93.
Hartman S, Freeman SM, Bales KL, Belsky J. Prenatal rtress as a risk-and an opportunity-factor. Psychol Sci. 2018;29:572–80.
Gueron-Sela N, Atzaba-Poria N, Meiri G, Marks K. The caregiving environment and developmental outcomes of preterm infants: diathesis stress or differential susceptibility effects? Child Dev. 2015;86:1014–30.
Slagt M, Dubas JS, Dekovic M, van Aken MA. Differences in sensitivity to parenting depending on child temperament: a meta-analysis. Psychol Bull. 2016;142:1068–110.
Aron E. The highly sensitive person: how to thrive when the world overwhelms You. Broadway Books; 1997.
Aron EN, Aron A, Jagiellowicz J. Sensory processing sensitivity: a review in the light of the evolution of biological responsivity. Pers Soc Psychol Rev. 2012;16:262–82.
Homberg JR, Schubert D, Asan E, Aron EN. Sensory processing sensitivity and serotonin gene variance: insights into mechanisms shaping environmental sensitivity. Neurosci Biobehav Rev. 2016;71:472–83.
Sih A, Bell AM. Insights for behavioral ecology from behavioral syndromes. Adv Study Behav. 2008;38:227–81.
Lionetti F, Aron A, Aron EN, Burns GL, Jagiellowicz J, Pluess M. Dandelions, tulips and orchids: evidence for the existence of low-sensitive, medium-sensitive and high-sensitive individuals. Transl Psychiatry. 2018;8:24.
Assary E, Zavos HMS, Krapohl E, Keers R, Pluess M. Genetic architecture of environmental sensitivity reflects multiple heritable components: a twin study with adolescents. Mol Psychiatry 2020; https://doi.org/10.1038/s41380-020-0783-8.
Chen C, Chen C, Moyzis R, Stern H, He Q, Li H, et al. Contributions of dopamine-related genes and environmental factors to highly sensitive personality: a multi-step neuronal system-level approach. PLoS ONE. 2011;6:e21636.
Licht C, Mortensen EL, Knudsen GM. Association between sensory processing sensitivity and the serotonin transporter polymorphism 5-HTTLPR short/short genotype. Biol Psychiatry. 2011;69:152S–3S.
Licht CL, Mortensen EL, Hjordt LV, Stenbaek DS, Arentzen TE, Nørremølle A, et al. Serotonin transporter gene (SLC6A4) variation and sensory processing sensitivity-Comparison with other anxiety-related temperamental dimensions. Mol Genet Genomic Med 2020;8:e1352.
Weyn S, Van Leeuwen K, Pluess M, Lionetti F, Greven CU, Goosens L, et al. Psychometric properties of the highly sensitive child scale across developmental stage, gender and country. Curr Psychol. 2019; https://doi.org/10.1007/s12144-19-00254-5.
Smith HLSJ, Erford BT. Clinical and research utility of the highly sensitive person scale. J Ment Health Counsel. 2019;41:221–41.
Bakker K, Moulding R. Sensory-processing sensitivity, dispositional mindfulness and negative psychological symptoms. Pers Individ Dif. 2012;53:341–6.
Brindle K, Moulding R, Bakker K, Nedeljkovic M. Is the relationship between sensory-processing sensitivity and negative affect mediated by emotional regulation? Aust J Psychol 2015; https://doi.org/10.1111/ajpy.12084.
Benham G, Woody EZ, Wilson KS, Nash MR. Expect the unexpected: ability, attitude, and responsiveness to hypnosis. J Pers Soc Psychol. 2006;91:342–50.
Liss M, Mailloux J, Erchull MJ. The relationship between sensory processing sensitivity, alexithymia, autism, depression, and anxiety. Pers Individ Dif. 2008;45:255–9.
Liss M, Timmel L, Baxley K, Killingsworth P. Sensory processing sensitivity and its relation to parental bonding, anxiety, and depression. Pers Individ Dif. 2005;39:1429–39.
Redfearn RAvIK, Stenmark CK. The impact of sensory processing sensitivity on stress and burnout in nurses. Int J Stress Manag. 2020;27:370–9.
Smolewska KA, McCabe SB, Woody EZ. A psychmetric evaluation of the highy sensitive person scale: the compenents of sensory processing sensitivity and their relation to the BIS/BAS and “Big Five”. Pers Individ Dif. 2006;40:1269–79.
Aron EN, Aron A. Sensory-processing sensitivity and its relation to introversion and emotionality. J Pers Soc Psychol. 1997;73:345–68.
Brohl AS, Van Leeuwen K, Pluess M, De Fruyt F, Bastin M, Weyn S, et al. First look at the five-factor model personality facet associations with sensory processing sensitivity. Curr Psychol 2020; https://doi.org/10.1007/s12144-020-00998-5.
Bridges D, Schendan HE. The sensitive, open creator. Pers Individ Diff. 2018;142, 179–85.
Harms R, Hatak I, Chang M. Sensory processing sensitivity and entrepreneurial intention: the strength of a weak trait. J Bus Ventur Insights. 2019;12:e00132.
Jagiellowicz J, Xu X, Aron A, Aron E, Cao G, Feng T, et al. The trait of sensory processing sensitivity and neural responses to changes in visual scenes. Soc Cogn Affect Neurosci. 2011;6:38–47.
Gerstenberg F. Sensory-processing sensitivity predicts performance on a visual search task followed by an increase in perceived stress. Pers Individ Dif. 2012;53:496–500.
Slagt M, Dubas JS, Denissen JJ, Decovik M, Van Aken MA. Sensory processing sensitivity as a marker of differential susceptibility to parenting. Dev Psychol. 2018;54:543–58.
Nocebtini AME, Pluess M. The personality trait of environmental sensitivity predicts children’s positive response to school-based antibullying intervention. Clin Psychol Sci. 2018;6:848–59.
Pluess M, Boniwell I. Sensory-processing sensitivity predicts treatment response to a school-based depression prevention program: evidence of vantage sensitivity. Pers Individ Dif. 2015;82:40–5.
Belsky J, van Ijzendoorn M. Genetic differential susceptibility to the effects of parenting. Curr Opin Psychol. 2017;15:125–30.
Smith SM, Fox PT, Miller KL, Glahn DC, Fox M, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–5.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506.
Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Carretie L. Exogenous (automatic) attention to emotional stimuli: a review. Cogn Affect Behav Neurosci. 2014;14:1228–58.
Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52.
Hermans EJ, Henckens MJ, Joels M, Fernandez G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37:304–14.
Gard AM, Shaw DS, Forbes EE, Hyde LW. Amygdala reactivity as a marker of differential susceptibility to socioeconomic resources during early adulthood. Dev Psychol. 2018;54:2341–55.
Klucken T, Wehrum S, Schweckendiek J, Merz CJ, Hennig J, Vaitl D, et al. The 5-HTTLPR polymorphism is associated with altered hemodynamic responses during appetitive conditioning. Hum Brain Mapp. 2013;34:2549–60.
Drabant EM, Ramel W, Edge MD, Hyde LW, Kuo JR, Goldin RR, et al. Neural mechanisms underlying 5-HTTLPR-related sensitivity to acute stress. Am J Psychiatry. 2012;169:397–405.
Johnson PL, Molosh AI, Federici LM, Bernabe C, Haggerty D, Fitz SD, et al. Assessment of fear and anxiety associated behaviors, physiology and neural circuits in rats with reduced serotonin transporter (SERT) levels. Transl Psychiatry. 2019;9:33.
Acevedo B, Jagiellowicz J, Aron E, Marhenke R, Aron A. Sensory processing sensitivity and childhood quality’s effects on neural responses to emotional stimuli. Clin Neuropsychiatry. 2017;14:359–73.
Johns CB, Lacadie C, Vohr B, Ment LR, Scheinost D. Amygdala functional connectivity is associated with social impairments in preterm born young adults. Neuroimage Clin. 2019;21:101626.
Javanbakht A, Tompson S, Kitayama S, King A, Yoon C, Liberzon I. Gene by culture effects on emotional processing of social cues among East Asians and European Americans. Behav Sci (Basel) 2018;8:62.
Redlich R, Schneider I, Kerkenberg N, Opel N, Bauhaus J, Enneking V, et al. The role of BDNF methylation and Val(66) Met in amygdala reactivity during emotion processing. Hum Brain Mapp. 2020;41:594–604.
Acevedo BP, Aron EN, Aron A, Sangster MD, Collins N, Brown LL. The highly sensitive brain: an fMRI study of sensory processing sensitivity and response to others’ emotions. Brain Behav. 2014;4:580–94.
Hong JS, Kim SM, Bae S, Han DH. Impulsive internet game play is associated with increased functional connectivity between the default mode and salience networks in depressed patients with short allele of serotonin transporter gene. Front Psychiatry. 2018;9:125.
Thomason ME, Yoo DJ, Glover GH, Gotlib IH. BDNF genotype modulates resting functional connectivity in children. Front Hum Neurosci. 2009;3:55.
Chen C, Xiu D, Chen C, et al. Regional homogeneity of resting-state brain activity suppresses the effect of dopamine-related genes on sensory processing sensitivity. PLoS ONE. 2015;10:e0133143.
Degnan AJ, Wisnowski JL, Choi S, Ceschin R, Bhushan C, Leahy RM, et al. Altered structural and functional connectivity in late preterm preadolescence: an anatomic seed-based study of resting state networks related to the posteromedial and lateral parietal cortex. PLoS ONE. 2015;10:e0130686.
Madsen MK, Mc Mahon B, Andersen SB, Siebner HR, Knudsen GM, Fisher PM. Threat-related amygdala functional connectivity is associated with 5-HTTLPR genotype and neuroticism. Soc Cogn Affect Neurosci. 2016;11:140–9.
Fang Z, Zhu S, Gillihan SJ, Korczykowski M, Detre JA, Rao H. Serotonin transporter genotype modulates functional connectivity between amygdala and PCC/PCu during mood recovery. Front Hum Neurosci. 2013;7:704.
White TP, Symington I, Castellanos NP, Brittain PJ, Walsh SF, Nam KW, et al. Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. Neuroimage Clin. 2014;4:352–65.
Cao W, Cao X, Hou C, Li T, Cheng Y, Jiang L, et al. Effects of cognitive training on resting-state functional connectivity of default mode, salience, and central executive networks. Front Aging Neurosci. 2016;8:70.
Klumpers F, Kroes MC, Heitland I, Everaerd D, Akkermans SEA, Oosting RS, et al. Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biol Psychiatry 2014;78:582-89.
Ernst LH, Lutz E, Ehlis AC, Fallgatter AJ, Reif A, Plichta MM. Genetic variation in MAOA modulates prefrontal cortical regulation of approach-avoidance reactions. Neuropsychobiology. 2013;67:168–80.
Loewenstern J, You X, Merchant J, Gordon EM, Stollstorff M, Devaney J, et al. Interactive effect of 5-HTTLPR and BDNF polymorphisms on amygdala intrinsic functional connectivity and anxiety. Psychiatry Res Neuroimaging. 2019;285:1–8.
Wehrle FM, Michels L, Guggenberger R, Huber R, Latal B, O’Gorman RL, et al. Altered resting-state functional connectivity in children and adolescents born very preterm short title. Neuroimage Clin. 2018;20:1148–56.
Kolesar TA, Bilevicius E, Wilson AD, Kornelsen J. Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. Neuroimage Clin. 2019;24:102016.
Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev. 2015;56:330–44.
Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76.
van der Meer D, Hoekstra PJ, Bralten J, Donkelaar M, Heslenfeld DJ, Oosterlaan J, et al. Interplay between stress response genes associated with attention-deficit hyperactivity disorder and brain volume. Genes Brain Behav. 2016;15:627–36.
Summerfield C, Egner T, Greene M, Koechlin E, Mangels J, Hirsch J. Predictive codes for forthcoming perception in the frontal cortex. Science. 2006;314:1311–4.
Fan J. An information theory account of cognitive control. Front Hum Neurosci. 2014;8:680.
Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–9.
Volf NV, Belousova LV, Knyazev GG, Kulikov AV. Gender differences in association between serotonin transporter gene polymorphism and resting-state EEG activity. Neuroscience. 2015;284:513–21.
Papousek I, Reiser EM, Schulter G, Fink A, Holmes EA, Niederstätter H, et al. Serotonin transporter genotype (5-HTTLPR) and electrocortical responses indicating the sensitivity to negative emotional cues. Emotion. 2013;13:1173–81.
Marusak HA, Furman DJ, Kuruvadi N, Shattuck DW, Joshi SH, Joshi AA, et al. Amygdala responses to salient social cues vary with oxytocin receptor genotype in youth. Neuropsychologia. 2015;79:1–9.
Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–93.
Beevers CG, Pacheco J, Clasen P, McGeary JE, Schnyer D. Prefrontal morphology, 5-HTTLPR polymorphism and biased attention for emotional stimuli. Genes Brain Behav. 2010;9:224–33.
Stollstorff M, Munakata Y, Jensen AP, et al. Individual differences in emotion-cognition interactions: emotional valence interacts with serotonin transporter genotype to influence brain systems involved in emotional reactivity and cognitive control. Front Hum Neurosci. 2013;7:327.
Nocentini A, Menesini E, Pluess M. Environmental sensitivity predicts treatment response to anti-bullying intervention: evidence of vantage sensitivity. Clin Psychol Sci. 2017;6:216770261878219.
McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. J Cogn Neurosci. 2010;22:248–62.
Pluess M, Boniwell I, Hefferon K, Tunariu A. Preliminary evaluation of a school-based resilience-promoting intervention in a high-risk population: application of an exploratory two-cohort treatment/control design. PLoS ONE. 2017;12:e0177191.
Jasinska AJ, Ho SS, Taylor SF, Burmeister M, Villafuerte S, Polk TA. Influence of threat and serotonin transporter genotype on interference effects. Front Psychol. 2012;3:139.
Morey RA, Hariri AR, Gold AL, et al. Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC Psychiatry. 2011;11:76.
Nonkes LJ, van de V II, de Leeuw MJ, Wijlaars LP, Maes JH, Homberg JR. Serotonin transporter knockout rats show improved strategy set-shifting and reduced latent inhibition. Learn Mem. 2012;19:190–3.
Dreisbach G, Muller J, Goschke T, et al. Dopamine and cognitive control: the influence of spontaneous eyeblink rate and dopamine gene polymorphisms on perseveration and distractibility. Behav Neurosci. 2005;119:483–90.
Azaefar AZY, Alishbayli A, Schubert D, Homberg JR, Celikel T Serotonergic development of active sensing. BioRxiv 2019; https://doi.org/10.1101/762534.
Markett S, Montag C, Walter NT, Plieger T, Reuter M. On the molecular genetics of flexibility: the case of task-switching, inhibitory control and genetic variants. Cogn Affect Behav Neurosci. 2011;11:644–51.
Borg J, Henningsson S, Saijo T, et al. Serotonin transporter genotype is associated with cognitive performance but not regional 5-HT1A receptor binding in humans. Int J Neuropsychopharmacol. 2009;12:783–92.
Miceli S, Nadif KN, Joosten J, et al. Reduced inhibition within layer IV of sert knockout rat barrel cortex is associated with faster sensory integration. Cereb Cortex 2017;27:933–49.
Bosia M, Anselmetti S, Pirovano A, et al. HTTLPR functional polymorphism in schizophrenia: executive functions vs. sustained attention dissociation. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:81–5.
Tukel R, Alkas E, Gurvit H, et al. Serotonin transporter promoter polymorphism is associated with executive function impairments in patients with obsessive compulsive disorder. Clin Neuropsychol. 2016;30:536–46.
Taren AA, Gianaros PJ, Greco CM, et al. Mindfulness meditation training and executive control network resting state functional connectivity: a randomized controlled trial. Psychosom Med. 2017;79:674–83.
Bauer CCC, Whitfield-Gabrieli S, Diaz JL, Pasaye EH, Barrios FA. From state-to-trait meditation: reconfiguration of central executive and default mode networks. eNeuro. 2019;6:ENEURO.0335-18.2019.
Takahashi TKI, Nitta Y, Kumano H. Dispositional mindfulness mediates the relationship between sensory-processing sensitivity and trait anxiety, well-being, and psychosomatic symptoms. Psychol Rep 2019;123:1083–98.
Fouragnan E, Queirazza F, Retzler C, Mullinger KJ, Philiastides MG. Spatiotemporal neural characterization of prediction error valence and surprise during reward learning in humans. Sci Rep. 2017;7:4762.
Knop J, van IMH, Bakermans-Kranenburg MJ, Joels M, van der Veen R. Maternal care of heterozygous dopamine receptor D4 knockout mice: Differential susceptibility to early-life rearing conditions. Genes Brain Behav 2020;19:e12655.
Giza JI, Kim J, Meyer HC, et al. The BDNF Val66Met prodomain disassembles dendritic spines altering fear extinction circuitry and behavior. Neuron. 2018;99:163–78.
Lallias D, Quillet E, Begout ML, et al. Genetic variability of environmental sensitivity revealed by phenotypic variation in body weight and (its) correlations to physiological and behavioral traits. PLoS ONE. 2017;12:e0189943.
Gozzi A, Schwarz AJ. Large-scale functional connectivity networks in the rodent brain. Neuroimage. 2016;127:496–509.
Jagiellowicz J, Aron A, Aron E. Relationship between the temperament trait of sensory processing sensitivity and emotional reactivity. Soc Behav Pers. 2016;44:185–200.
Acknowledgements
We thank research assistant Madeleine Ghazarian for helping with the literature search. This work was supported by the Dutch Research Council through the ERANID Grant “STANDUP” awarded to JH. The Dutch Research Council had no role in the creation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Homberg, J.R., Jagiellowicz, J. A neural model of vulnerability and resilience to stress-related disorders linked to differential susceptibility. Mol Psychiatry 27, 514–524 (2022). https://doi.org/10.1038/s41380-021-01047-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01047-8
This article is cited by
-
Unique Psychological Mechanisms Underlying Psilocybin Therapy Versus Escitalopram Treatment in the Treatment of Major Depressive Disorder
International Journal of Mental Health and Addiction (2024)
-
Case analysis of long-term negative psychological responses to psychedelics
Scientific Reports (2023)
-
Overlapping brain correlates of superior cognition among children at genetic risk for Alzheimer’s disease and/or major depressive disorder
Scientific Reports (2023)