Abstract
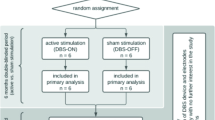
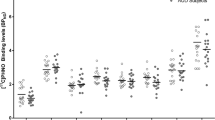
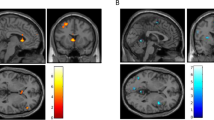
Alcohol use disorder (AUD) is a highly prevalent, often refractory, medical illness. The symptoms of AUD are driven by dysfunction in several neurocircuits centered on the nucleus accumbens (NAc). Case reports and animal studies suggest NAc-DBS may be an effective harm-reduction treatment in severe AUD. Six patients with severe, refractory AUD underwent NAc-DBS. Safety metrics and clinical outcomes were recorded. Positron emission tomography (FDG-PET) was used to measure glucose metabolism in the NAc at baseline and 6 months. Functional magnetic resonance imaging (fMRI) was used to characterize postoperative changes in NAc functional connectivity to the rest of the brain, as well as NAc and dorsal striatal reactivity to alcoholic visual cues. This study was registered with ClinicalTrials.gov, NCT03660124. All patients experienced a reduction in craving. There was a significant reduction in alcohol consumption, alcohol-related compulsivity, and anxiety at 12 months. There was no significant change in depression. FDG-PET analysis demonstrated reduced NAc metabolism by 6 months, which correlated with improvements in compulsive drinking behaviors. Clinical improvement correlated with reduced functional connectivity between the NAc and the visual association cortex. Active DBS was associated with reduced activation of the dorsal striatum during passive viewing of alcohol-containing pictures. NAc-DBS is feasible and safe in patients with severe, otherwise refractory AUD. It is associated with a reduction in cravings and addictive behavior. A potential mechanism underlying this process is a down-regulation of the NAc, a disruption of its functional connectivity to the visual association cortex, and interference of cue-elicited dorsal striatum reactivity. Trial Registration NCT03660124 (www.clinicaltrials.gov).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data presented in this article will be available to other researchers upon reasonable request to the corresponding author.
References
Schuckit MA. Remarkable increases in alcohol use disorders. JAMA Psychiatry. 2017;74:869–70.
Rehm J, Gnam W, Popova S, Baliunas D, Brochu S, Fischer B, et al. The costs of alcohol, illegal drugs, and tobacco in Canada, 2002. J Stud Alcohol Drugs. 2007;68:886–95.
Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry. 2015;72:757–66.
George TP. Alcohol use and misuse. CMAJ. 2007;176:621–2.
Poznyak V, Rekve D. Global status report on alcohol and health 2014. Geneva: World Health Organization; 2014.
Westman J, Wahlbeck K, Laursen TM, Gissler M, Nordentoft M, Hallgren J, et al. Mortality and life expectancy of people with alcohol use disorder in Denmark, Finland and Sweden. Acta Psychiatr Scand. 2015;131:297–306.
Schwarzinger M, Thiebaut SP, Baillot S, Mallet V, Rehm J. Alcohol use disorders and associated chronic disease—a national retrospective cohort study from France. BMC Public Health. 2017;18:43.
World Health Organization. WHO global status report on alcohol 2004. Geneva: World Health Organization; 2004.
Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73.
Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47.
Park YS, Sammartino F, Young NA, Corrigan J, Krishna V, Rezai AR. Anatomic review of the ventral capsule/ventral striatum and the nucleus accumbens to guide target selection for deep brain stimulation for obsessive-compulsive disorder. World Neurosurg. 2019;126:1–10.
Denys D, Graat I, Mocking R, de Koning P, Vulink N, Figee M, et al. Efficacy of deep brain stimulation of the ventral anterior limb of the internal capsule for refractory obsessive-compulsive disorder: a clinical cohort of 70 patients. Am J Psychiatry. 2020;177:265–71.
Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 2012;37:1975–85.
Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77:406–24.
Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15:148–60.
Mayberg HS, Riva-Posse P, Crowell AL. Deep brain stimulation for depression: keeping an eye on a moving target. JAMA Psychiatry. 2016;73:439–40.
Kuhn J, Lenartz D, Huff W, Lee S, Koulousakis A, Klosterkoetter J, et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry. 2007;78:1152–3.
Muller UJ, Sturm V, Voges J, Heinze HJ, Galazky I, Buntjen L, et al. Nucleus accumbens deep brain stimulation for alcohol addiction—safety and clinical long-term results of a pilot trial. Pharmacopsychiatry. 2016;49:170–3.
Hassan O, Phan S, Wiecks N, Joaquin C, Bondarenko V. Outcomes of deep brain stimulation surgery for substance use disorder: a systematic review. Neurosurg Rev. 2021;44:1967–76.
wennberg P. The timeline follow back technique: psychometric properties of a 28-day timeline for measuring alcohol consumption. Ger J Psychiatry. 1998;2:62–8.
Volk RJ, Steinbauer JR, Cantor SB, Holzer CE 3rd. The alcohol use disorders identification test (AUDIT) as a screen for at-risk drinking in primary care patients of different racial/ethnic backgrounds. Addiction. 1997;92:197–206.
Anton RF, Moak DH, Latham P. The obsessive compulsive drinking scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–9.
Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–6.
Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209.
Hamilton M. Standardised assessment and recording of depressive symptoms. Psychiatr Neurol Neurochir. 1969;72:201–5.
Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7.
Beck AT, Steer RA. Internal consistencies of the original and revised beck depression inventory. J Clin Psychol. 1984;40:1365–7.
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–90.
Davidson B, Tam F, Yang B, Meng Y, Hamani C, Graham SJ, et al. Three-tesla magnetic resonance imaging of patients with deep brain stimulators: results from a phantom study and a pilot study in patients. Neurosurgery. 2020;88:349–55.
Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41.
Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, et al. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–9.
Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–86.
Spies C, Tonnesen H, Andreasson S, Helander A, Conigrave K. Perioperative morbidity and mortality in chronic alcoholic patients. Alcohol Clin Exp Res. 2001;25:164S–70S.
Voges J, Muller U, Bogerts B, Munte T, Heinze HJ. Deep brain stimulation surgery for alcohol addiction. World Neurosurg. 2013;80:S28.e1–31.
Muller UJ, Sturm V, Voges J, Heinze HJ, Galazky I, Heldmann M, et al. Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: first experience with three cases. Pharmacopsychiatry. 2009;42:288–91.
Buchsbaum MS, Bralet M. FDG-PET scans in patients with alcohol use disorder. Lisbon: WCP-2019; 2019.
Kohno M, Dennis LE, McCready H, Hoffman WF. Executive control and striatal resting-state network interact with risk factors to influence treatment outcomes in alcohol-use disorder. Front Psychiatry. 2017;8:182.
Bach P, Reinhard I, Buhler S, Vollstadt-Klein S, Kiefer F, Koopmann A. Oxytocin modulates alcohol-cue induced functional connectivity in the nucleus accumbens of social drinkers. Psychoneuroendocrinology. 2019;109:104385.
Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–53.
Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–70.
Pool JL. Psychosurgery in older people. J Am Geriatr Soc. 1954;2:456–66.
Cheung SW, Racine CA, Henderson-Sabes J, Demopoulos C, Molinaro AM, Heath S, et al. Phase I trial of caudate deep brain stimulation for treatment-resistant tinnitus. J Neurosurg. 2019:1–10. Online ahead of print.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60.
Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354:1526.
Lipsman N, Woodside DB, Giacobbe P, Hamani C, Carter JC, Norwood SJ, et al. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lancet. 2013;381:1361–70.
Davidson B, Hamani C, Rabin JS, Goubran M, Meng Y, Huang Y, et al. Magnetic resonance-guided focused ultrasound capsulotomy for refractory obsessive compulsive disorder and major depressive disorder: clinical and imaging results from two phase I trials. Mol Psychiatry. 2020;25:1946–57.
Mantione M, Nieman DH, Figee M, Denys D. Cognitive-behavioural therapy augments the effects of deep brain stimulation in obsessive-compulsive disorder. Psychol Med. 2014;44:3515–22.
Acknowledgements
Dr. Josee Lynch made important contributions during early stages of project conception. Dr. Sabine Vollstädt-Klein generously shared the alcohol visual-cue dataset used in the fMRI portion of the study.
Funding
Funding was supplied by internal research funds, as well as support from the Harquail Centre for Neuromodulation, and philanthropic support to the Sunnybrook Foundation—none of whom played any role in the collection or interpretation of data, writing of the manuscript, or the decision to publish.
Author information
Authors and Affiliations
Contributions
BD made significant contributions to conceptualization, data curation, formal analysis, methodology, validation, visualization, writing (original draft) and writing (review/editing). He verified the underlying data. PG and TPG made significant contributions to conceptualization, funding acquisition, methodology, project administration, resources, supervision, and writing (review/editing). SMN made significant contributions to data curation, methodology, project administration, resources, validation, and writing (review/editing). JSR made significant contributions to data curation, formal analysis, methodology, project administration, resources, supervision, validation, writing (original draft) and writing (review/editing). AJN made significant contributions to data curation, formal analysis, project administration, resources, software, writing (original draft), and writing (review/editing). MG made significant contributions to formal analysis, methodology, project administration, resources, software, supervision, visualization, writing (original draft), and writing (review/editing). AB made significant contributions to conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, resources, software, supervision, validation, visualization, writing (original draft) and writing (review/editing). She verified the underlying data. YM made significant contributions to conceptualization, data curation, formal analysis, project administration, resources, software, validation, writing (original draft), and writing (review/editing). CBP made significant contributions to data curation, formal analysis project administration, resources, software, validation, and writing (review/editing). SJG made significant contributions to conceptualization, data curation, funding acquisition, methodology, project administration, resources, software, supervision, and writing (review/editing). FT made significant contributions to conceptualization, data curation, formal analysis, methodology, resources, software, validation, and writing (review/editing). CH made significant contributions to conceptualization, funding acquisition, methodology, project administration, resources, software, supervision, validation, visualization, writing (original draft) and writing (review/editing). He verified the underlying data. NL made significant contributions to conceptualization, funding acquisition, methodology, project administration, resources, software, supervision, validation, visualization, writing (original draft) and writing (review/editing). He verified the underlying data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Davidson, B., Giacobbe, P., George, T.P. et al. Deep brain stimulation of the nucleus accumbens in the treatment of severe alcohol use disorder: a phase I pilot trial. Mol Psychiatry 27, 3992–4000 (2022). https://doi.org/10.1038/s41380-022-01677-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01677-6
This article is cited by
-
A systematic review and meta-analysis of neuromodulation therapies for substance use disorders
Neuropsychopharmacology (2024)
-
Deep brain stimulation for psychostimulant use disorders
Journal of Neural Transmission (2024)
-
Abnormal functional connectivity of the nucleus accumbens subregions mediates the association between anhedonia and major depressive disorder
BMC Psychiatry (2023)
-
Deep brain stimulation of the nucleus accumbens in treatment-resistant alcohol use disorder: a double-blind randomized controlled multi-center trial
Translational Psychiatry (2023)
-
Mehr abstinente Tage nach THS
InFo Neurologie + Psychiatrie (2023)