Abstract
Extensive evidence points to a role for GABAergic signaling in the amygdala in mediating the effects of alcohol, including presynaptic changes in GABA release, suggesting effects on GABAergic neurons. However, the majority of studies focus solely on the effects of alcohol on principal neurons. Here we demonstrate that δ-GABAARs, which have been suggested to confer ethanol sensitivity, are expressed at a high density on parvalbumin (PV) interneurons in the basolateral amygdala (BLA). Thus, we hypothesized that δ-GABAARs on PV interneurons may represent both an initial pharmacological target for alcohol and a site for plasticity associated with the expression of various behavioral maladaptations during withdrawal from binge drinking. To investigate this, we used a mouse model of voluntary alcohol intake (Drinking-in-the-Dark-Multiple Scheduled Access) to induce escalating heavy binge drinking and anxiety-like behavior in mice. This pattern of intake was associated with increased δ protein expression on parvalbumin positive interneurons in both the BLA and hippocampus. Loss of δ-GABAARs specifically in PV interneurons (PV:δ−/−) increased binge drinking behavior, reduced sensitivity to alcohol-induced motor incoordination, enhanced sensitivity to alcohol-induced hyperlocomotion and blocked the expression of withdrawal from binge drinking. This study is the first to demonstrate a role for δGABAARs specifically in PV-expressing interneurons in modulating binge alcohol intake and withdrawal-induced anxiety.
Similar content being viewed by others
Introduction
Binge drinking is a dangerous pattern of alcohol consumption that is increasing in prevalence across the world [1]. This is of concern, given the strong association between heavy episodic drinking and the development of alcohol use disorders. Though only 1 in 9 of these binge drinkers will be diagnosed with an alcohol use disorder [2], this pattern of heavy episodic use is also implicated in the development of negative affect and anxiety [3, 4]. Preclinical findings also support the development of anxiety-like behavior during withdrawal from this pattern of alcohol use [5,6,7]. Given the increasing prevalence of binge drinking, there is a critical need to clarify the biological underpinnings of this type of alcohol intake.
The GABAergic system has long been considered a relevant molecular target for alcohol, given the GABAmimmetic characteristic of many of alcohol’s effects, including changes in motor coordination, locomotion and anxiety. Indeed, concentrations of ethanol relevant to an active heavy binge drinking session (i.e., 17–30 mM) have been shown to augment tonic inhibition mediated by extrasynaptic GABAA receptors [8,9,10,11,12,13,14,15,16]. Consistent with a role for δ-GABAARs in mediating the effects of alcohol, both genetic [17] and pharmacological [18] manipulation of these receptors has been shown to alter alcohol drinking behaviors. Specific to binge drinking, activation of δ-GABAARs using THIP has been shown to reduce this pattern of alcohol intake in mice [19,20,21]. The well-established relationship between neurosteroids that modulate δ-GABAA receptor activity and alcohol [22,23,24], also supports a role for δ-GABAARs as a neural substrate for the behavioral effects associated with this pattern of alcohol use.
A number of independent labs have found long-lasting, functionally relevant δ- GABAAR adaptations associated with alcohol exposure. Together, their efforts suggest that persistent δ-GABAAR adaptations may be seen following exposure to alcohol during adolescence [14, 25,26,27], or alcohol exposure that leads to significant as seen in chronic intermittent ethanol (CIE) paradigms [12, 13, 28]. This persistent downregulation of δ mRNA and protein is even found in the postmortem brains of alcohol-dependent patients [29]. Importantly, though this downregulation in δ-GABAA is seen following just a single exposure to ethanol, this plasticity is short-lived [30]. This transience raises a question about the role adaptations in this system may play in the relationship between binge drinking and the development of problems with alcohol use and whether δ-GABAAR plasticity is most relevant after multiple cycles of exposure and withdrawal has transitioned the adult binger from restrained and irregular use to an alcohol use disorder.
Interestingly, much of the preclinical work assessing the role for δ-GABAARs in mediating the effects of low to moderate alcohol doses have focused on δ-GABAARs expressed by principal cells. Though excitatory cells represent the major population of neurons in many circuits relevant to reward and intoxication, inhibitory interneurons play a critical role in regulating the activity of these principal cells and, therefore, may also play a role in mediating the effects of alcohol.
In support of this hypothesis, the GABA receptor composition in interneurons, which incorporate the δ subunit, has been demonstrated to confer unique sensitivity to low dose ethanol in the hippocampal dentate gyrus [10]. In the hippocampal dentate gyrus, all parvalbumin positive interneurons express δ containing GABAA receptors [31], while less than 10% of somatostatin interneurons and no calbindin or calretinin positive interneurons expresses this GABAA receptor subtype. The increased sensitivity to ethanol’s effects on tonic inhibition in the molecular layer of the hippocampal dentate may involve interneurons expressing unique δ-GABAA receptors [10]. Interneurons in the basolateral amygdala (BLA), a region known to express interneurons that play a role in GABAergic regulation of anxiety-like behavior [32], may also express these δ-GABAA receptors[31]. Indeed, elegant work in the central amygdala(CeA), an amygdalar sub-nucleus with a significant population of interneurons, demonstrates that subpopulations of neurons in the CeA expressing δ- GABAA receptors have an enhanced sensitivity to ethanol effects on tonic current [33] that may be altered by chronic alcohol exposure [34]. Though present evidence supports changes in the BLA and anxiety associated with alcohol dependence [35], it is unclear whether regulation of δ-GABAA receptors in this region is involved in the development of anxiety-like behavior following binge.
Based on these findings, we hypothesized that δ-GABAARs on interneurons play a role in mediating the effects of ethanol exposure seen following voluntary consumption. To test this hypothesis, we investigated changes in the expression of δ-GABAARs on parvalbumin positive interneurons (PV + INs) following binge drinking. To further support a role for δ-GABAARs on PV + INs in binge drinking and its behavioral consequences, we utilized mice with selective deletion of δ on PV interneurons (PV:δ−/−). Here we demonstrate that these mice consume more alcohol in a limited-access paradigm that models binge-like consumption, display less intoxication to alcohol, and display reduced anxiety-like behaviors during withdrawal from alcohol. These data highlight δ-GABAARs on PV + INs as an important target for alcohol and support a role in both the maintenance and consequences of binge alcohol consumption.
Methods
Animals
Adult (>P60) male and female mice were housed at the Tufts University School of Medicine in a temperature and humidity controlled environment on a reversed 12 h light/dark cycle. Food was available ad libitum in the home cage. Water was available ad libitum in the home cage except when ethanol was made available (see Drinking-in-the-Dark-Multiple Scheduled Access protocol, below). Animals were handled according to protocols approved by the Tufts University Institutional Animal Care and Use Committee.
Animals with parvalbumin-specific deletion of the GABAA-δ subunit (PV:δ−/−) were generated by crossing floxed Gabrd mice [36] with PV-Cre mice obtained from Jackson’s Lab (Stock #012358). These PV:δ−/− mice have recently been characterized [37, 38]. Genotyping for PV-Cre was performed in-house using the primers listed below. Genotyping for the floxed Gabrd was performed in-house as previously described [36] with the primers listed below to distinguish the wild-type Gabrd (449 bp) or floxed-Gabrd (543 bp) product.
PV-Cre:
5′: CATGAGGAGTGGCATACACG
3′: TTCGCGATTTGAGGTCTTCT
Gabrd:
5′: GACTCCAGTTGCCAAGCCTTTAATTCC
3′: CATCTGCCTGTACCTCCAATGCCTG
DID-MSA
To induce a pattern of escalating binge drinking, the Drinking-in-the-Dark-Multiple Scheduled Access (DID-MSA) procedure was adapted from refs. [39, 40]. Briefly, beginning at lights out, mice received access to water or a 20% unsweetened alcohol solution (95% ethanol Pharmco Products Inc., Brook- field, CT) during three, 1-h drinking sessions. Each drinking session was separated by 2-h breaks where regular water bottles were returned. Volumes consumed were recorded for each 1 h binge-drinking session and summed for the day.
Sucrose preference test
A separate cohort of PV:δ−/− and wild-type mice received 24 h access to sucrose (2% w/v) and water in a two-bottle choice paradigm for 4 days. Fluids were delivered in modified 50 mL conical tubes fitted with balled bearing sippers and were weighed at the same time (ZT 15–16) daily. Tube positions were rotated after 2 days to account for potential side preferences. Sucrose preference was calculated as change in sucrose bottle weight over sum of sucrose and water bottle weight changes (gsucrose/gsucrose + gwater volume (mL) of water consumed was also calculated for each individual mouse (1 gH2O = 1 mLH2O).
Balance beam
To measure intoxication, mice were trained to traverse a balance beam (122 cm long × 2 cm wide × 4 cm tall). Following the final drinking session on the final day of alcohol access (experiment 2) or 10 min following an intraperitoneal (i.p.) injection of 1.75 g/kg ethanol (experiment 3), each mouse traversed the same beam and the number of hind footslips were recorded.
Open field activity
To measure the locomotor response to ethanol, mice were administered an i.p. injection of saline or ethanol (20% v/v) and tested for 10 min in an open field assay as previously described [41]. Briefly, mice were placed in the center of a 40.64 × 40.64 cm open field photobeam frame lined with 16 photocells spaced 2.54 cm apart (Hamilton-Kinder). Photocell beam-breaks were measured using MotorMonitor software (Hamilton-Kinder) to determine entries into the center zone (20.32 × 20.32 cm square in center of maze) and distance traveled (cm).
Light/dark box
This light/dark box test was performed as previously described [41]. Mice were placed in the dark compartment of a two chambered box enclosed by a 22 × 43 cm photobeam frame with 8 equally spaced photocells (Hamilton-Kinder interfacing with MotorMonitor software) with the open compartment illuminated by a red bulb lamp. The distance traveled in the light compartment and emergence delay was measured using MotorMonitor software.
Blood ethanol concentration
For mice in experiments 3, submandibular blood samples were collected at the end of the balance beam test (within 12 min of 1.75 g/kg ethanol (20%v/v; i.p). Blood samples were collected via submandibular bleeds into heparinized tubes and centrifuged before storing plasma supernatant at −20 °C. Samples were later analyzed using an Analox Alcohol Analyzer (AM1, Analox Instruments, Lunenburg, MA) and accompanying reagents as described by manufacturer.
Immunohistochemistry
Immunohistochemistry was performed as previously described [42]. Twenty-four hours following behavioral testing, mice were anesthetized with isoflurane and euthanized by rapid decapitation. Brains were removed and immersion fixed by overnight incubation (at 4 °C) in 4% paraformaldehyde. Brains were cryoprotected using overnight incubations in 10 and 30% sucrose, snap frozen in dry-ice chilled isopentane, and maintained at −80 °C until cryosectioning.
For δ immunolabeling with DAB, endogenous peroxidase was quenched in free-floating 40 μm coronal sections with 3%H2O2 in methanol for 30 min then incubated in 0.05 M citrate buffer at 90 °C for 45 min to improve antigen retrieval. Slices were blocked in 10% normal goat serum in 1X PBS with 0.3% Triton X-100 for 1 h then incubated with a 1° antibody against δ-GABAAR (1:250, Millipore AB9752) overnight at 4 °C followed by a 2-h incubation with a biotinylated anti-rabbit antibody and a HRP-conjugated streptavidin (ABC Elite, Vector Laboratories). Immunolabeling was visualized with diaminobenzidine (DAB) and quantified by brightfield microscopy by measuring optical density in the region of interest using Image J-based FIJI software [43]. The tracer tool was used to outline a given region (Basolateral amygdala or hippocampal dentate gyrus) and total optical density was recorded using the software’s results manager and saved to Excel for later analysis.
For parvalbumin and δ co-immunofluorescence, free floating 40 μm coronal sections were incubated in 0.5M citrate buffer for 45 min at 90 °C. Slices were blocked in 10% normal goat serum in 1X PBS with 0.3% Triton X-100 for 1 h, then incubated with 1° antibodies against δ-GABAAR (1:250, Millipore AB9752) and parvalbumin (1:1000, Sigma P3088) for 4 days at 4 °C. Slices were then incubated with a biotinylated goat anti-rabbit (1:1000, Vector Laboratories BA1000) and Alexa-Fluor 647 conjugated goat anti mouse (1:200,ThermoFisher Scientific A28181) and Alexa-Fluor 488-streptavidin (1:200, ThermoFisher Scientific S32354). Immunolabeling was visualized on a Nikon a1r confocal and quantified using Image J software. A series of 1 μm step images were acquired using a ×40 oil-immersion objective and the same laser power and camera settings for samples across each treatment group. The 1 μm step containing the highest immunoreactivity in the channel corresponding to PV was used to identify PV positive cells and to verify that the δ co-immunofluorescence measured in the respective channel corresponds to that same cell. Specifically, cell bodies within a stack series were traced and saved to the ROI manager and the multi-measure tool was used to measure immunoreactivity in all steps in the stack for later assessment. For analysis, the cell/stack with the highest PV immunoreactivity was used to determine the co-expression of δ, reported as integrated optical density to account for any changes in cell size across cells selected.
Corticosterone ELISA
For mice in experiment 2, corticosterone (CORT) was quantified as previously described [41]. Briefly, following behavioral testing, trunk bloods were collected and centrifuged to isolate plasma. Duplicate 5 μL samples were assayed using an enzyme immunoassay according to manufacturer’s specifications (Enzo Life Sciences, Farmingdale, New York) and compared to a standard curve of known CORT concentrations.
Statistical measures
Data were analyzed using Graphpad Prism 7. A linear regression was used to assess escalation in binge drinking across days of access. Student’s t-test was used to compare the effects of binge drinking history (on anxiety-like behavior, δ expression) across groups (ethanol vs. water). For locomotor activity, area under the curve was generated to reflect changes in the distance traveled in each 1 min bout across the 10-min test. To assess the effects of genotype (PV:δ−/− vs. wild type) on binge drinking behavior, anxiety-like behavior, intoxication or locomotor activity, a one-way ANOVA was used within each sex. Bonferroni adjusted post hoc analyses or Dunnett’s post hoc tests were used to compare across all groups or to compare groups to an ultimate control (saline treatment, for example) as indicated. For all tests, p < 0.05 was used for significance.
Results
Experiment 1: Are changes in δ expression associated with escalated binge drinking and anxiety-like behavior during early withdrawal?
Binge drinking escalates using DID-MSA
Mice received 14 days of limited access to alcohol in a manner shown to produce escalated intake. Over the 14 days of access, females escalated their binge drinking, with a significant linear relationship between day and amount consumed [r2 = 0.88, p < 0.0001; Fig. 1b]. Bonferroni adjusted planned comparisons were used to assess degree of change in drinking behavior over the 2 weeks of access (Fig. 1c). By the end of the first week of drinking (day 7) mice increased the amount of alcohol they consumed in hourly binge 1 by 260% [t(20) = 2.96; p < 0.05], hourly binge 2 by 575% [t(20) = 5.42; p < 0.0001] and hourly binge 3 by 532% [t(20) = 5.5; p < 0.0001).
Escalated heavy binge drinking is associated with anxiety-like behavior during early withdrawal and enhanced δ protein expression in the basolateral amygdala. a Female C57BL/6J mice had 2 weeks of access to alcohol in three separate hourly binge sessions (DID-MSA) and 1 day of forced abstinence before testing for anxiety-like behavior using the light/dark box. Brains were collected the day following behavioral testing. b Mice steadily increased the total ethanol consumed during the DID-MSA protocol across two weeks (r2 = 0.88, p < 0.0001, n = 6 mice). c This enhanced intake involved increases in the hourly consumption of alcohol by day 7 (*p < 0.05, **p = <0.001 as compared to hourly intake from day 1) and modest enhancement in the final binge session by day 14. d There was no change in exploration of the dark side following binge drinking. e There was a significant increase in the time to emerge from the dark side of the box following binge drinking (t(9) = 2.37, *p < 0.05, n = 6 mice per treatment group). Photomicrographs of δ protein immunoreactivity in the hippocampal dentate gyrus (f) and BLA (i). Overall optical density of δ subunit expression remains unchanged in the molecular or granule cell layer (g), and the BLA (h) in mice with a 2 week binge drinking history
Early withdrawal from escalated binge drinking associated with anxiety-like behavior
At the end of the 14-day binge drinking period, mice were given 1 day of forced abstinence before testing for anxiety-like behavior during early withdrawal using the light dark box. There was no significant difference in activity in the dark side of the box (ratio of “dark side distance traveled” to “total distance traveled in the apparatus”; Fig. 1d) between ethanol
[M = 50.27% ± 1.17] or water drinkers [M = 51.3 ± 2.45]. Analysis of the secondary index of anxiety, emergence delay (Fig. 1e), showed that binge ethanol drinkers [M = 22 ± 3.44] took significantly longer to emerge from the dark side of the box at the start of the test, as compared to water drinking controls [M = 11.17 ± 3.05; t(9) = 2.37, p < 0.05].
Escalated binge drinking does not change overall δ expression in the hippocampus or basolateral amygdala
Following anxiety testing, mice were returned to their home-cages and brains harvested the following day (72 h following final binge) for immunohistochemical analysis as described. In the hippocampus, overall δ expression was not significantly changed by binge drinking history in either the molecular layer [water M = 99.81 ± 10.91., ethanol M = 90.51 ± 1.52, p = 0.51; n.s.], or granule cell layer [water M = 100.06 ± 8.75, ethanol M = 92.90 ± 7.79; p = 0.58; Fig. 1g] of the dentate gyrus. Similarly, there was no significant change in the overall expression of δ protein in the BLA of ethanol drinkers [M = 107.88 ± 3.88] when compared to controls [M = 100.7 ± 3.66].
Escalated binge drinking associated with specific increase of δ protein on parvalbumin interneurons (PV + INs) in hippocampus and basolateral amygdala
We did a cell body restricted assessment of δ protein expression co-localized on parvalbumin positive interneurons in confocal images of the hippocampal dentate gyrus and BLA. In the hippocampus, we found a significant increase in δ expressed on PV + INs in the hippocampus [t(53) = 4.1, p < 0.001; Fig. 2c] of ethanol drinkers [M = 274,459 ± 24,290 IOD] when compared to water drinking controls [M = 164,675 ± 13523 IOD]. Similarly, in the BLA we found a significant increase in δ expressed by PV + INs [t(22) = 2.67, p < 0.05; Fig. 2f] of ethanol drinkers [ethanol M = 158,275 ± 13,995 IOD] when compared to water controls [M = 85,524 ± 26,643 IOD]. Parvalbumin expression did not change in the BLA (Fig. 2f; t(4) = 0.46; p = 0.66) or hippocampus (Fig. 2f) as a function of ethanol exposure.
Binge drinking increases δ expression on parvalbumin positive interneurons in the hippocampus and basolateral amygdala. a Photomicrographs of DAPI (blue), δ (green) and parvalbumin (red) immunoreactivity in the hippocampal dentate gyrus of water (top) or ethanol (bottom) drinkers. b There was an increase in δ immunoreactivity in parvalbumin positive interneurons in the hippocampal dentate gyrus in ethanol drinkers (t(53) = 4.1, p < 0.001; n = 26–29 cells per treatment group). c There was no significant change in parvalbumin expression levels in the same cells d. Photomicrographs of DAPI (blue), δ (green), and parvalbumin (red) immunoreactivity in the basolateral amygdala of water (top) or ethanol (bottom) drinkers. e There was an increase in δ immunoreactivity in parvalbumin positive cells in the basolateral amygdala in ethanol drinkers (*p < 0.05, 9–15 cells per treatment group). f There was no significant change in parvalbumin in the same cells
Experiment 2: Are δ-GABAARs on PV-positive interneurons a necessary target for binge drinking?
Males with PV-specific knockout of GABAA-δ binge drink more ethanol
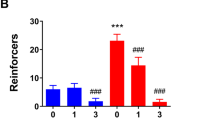
Mice were generated with PV + IN-specific deletion of the GABAAR δ subunit. These PV:δ−/− mice and their wild-type PV:δ+/+(WT) littermates were singly housed as adults and received limited-access to alcohol or water using DID-MSA protocol for 2 weeks. The total daily alcohol consumed across the 14 days of access is shown in Fig. 3. Females increased drinking over time [F(13,286) = 18.86, p < 0.0001; Fig. 3b] but showed no effect of genotype. In contrast, males also increased their drinking over time [F(1,13) = 12.57, p < 0.0001; Fig. 3d], but did show a significant effect of genotype on intake [F(1,13) = 5.03, p < 0.05] across these days. An assessment of the average ethanol consumed over this two week period showed that PV:δ−/− males (M = 4.84 ± 0.52, n = 7) consumed over 40% more alcohol than their wild-type counterparts (M = 3.44 ± 0.40, n = 9) during these 2 weeks of access [t(14) = 2.16, p < 0.05; Fig. 3e]. A separate cohort of mice given 24 h access to sucrose (2% w/v) and water for 4 days showed no significant effect of genotype on sucrose preference (Fig. 3f–h). Female PV:δ−/− had a similar preference for sucrose (M = 73.33 ± 8.06, n = 6) as wild-type females (73.55 ± 4.57, n = 6). Male PV:δ−/− mice also had similar preference for sucrose (M = 61.09 ± 16.43, n = 6) than wild-type males (M = 76.5 ± 7.13, n = 6; p = 0.41).
Parvalbumin selective deletion of δ GABAA receptor subunit associated with enhanced drinking in males. a Mice had access to ethanol or water using the DID-MSA protocol. b Females enhanced intake over days similarly across genotypes c. Females consumed similar amounts of alcohol across the access period(n = 8–9 per genotype). d Males enhanced drinking over the 2 weeks (F(1,13) = 12.57, p < 0.0001) but PV:δ−/− males showed greater daily intake (F(1,13) = 5.03, p < 0.05). e Average intake across the 2 weeks of access was significantly greater for PV:δ−/− males (t(14) = 2.16, *p < 0.05, n = 7–8 per genotype). f A separate cohort of mice has 24 h access to two bottles, one containing sucrose (2% w/v) or water for 4 days. g Females did not show any effect of genotype on the average sucrose preference over these 4 days. h Males did not show any significant effect of genotype on preference for sucrose the average sucrose preference over these 4 days
Males with parvalbumin-specific knockout of GABAA-δ are less sensitive to motor incoordination associated with binge drinking
Footslips on the balance beam following the final binge session were used as an index of motor incoordination. As our findings confirmed no difference in footslips between the genotypes at baseline, these data were consolidated for water drinkers and a one-way analysis was used to assess data. Females showed no effect of group on the number of footslips measured on the balance beam on this day (water M = 1.78 ± 0.26, n = 23; wild-type bingers M = 2.6 ± 0.72, n = 10; PV:δ−/− M = 3 ± 0.67, n = 9; Fig. 4b). Males showed a significant effect of group on footslips following the final access of alcohol or water [F(2,27) = 16.15, p < 0.0001; Fig. 4e]. Wild-type males (M = 3.67 ± 0.62, n = 9) were significantly intoxicated on this day of access, demonstrating an increase in the number of footslips; whereas, PV:δ−/− males that drank ethanol (M = 1.29 ± 0.29, n = 7) did not exhibit a difference in footslips compared to water drinking controls (M = 0.79 ± 0.21, n = 14).
PV:δ−/− mice are less sensitive to the sedative effects but more sensitive to the stimulant effects of alcohol. a Mice either had access to ethanol (or water) using DID-MSA for 14 days and were tested on the balance beam immediately following the final binge session or naive mice were administered ethanol placed in an open field for 10 min to assess locomotor activity and tested on the balance beam to assess motor incoordination. b Females did not show significant increases in footslips following binge drinking (p = 0.15; n.s.). c Following acute administration of 1.75 g/kg ethanol, wild-type females show increased activity compared to baseline for the first 3 min (p < 0.05 when compared to saline) following drug infusion, while PV:δ−/− females maintain a stimulant response for twice as long (p < 0.05 when compared to saline) and similarly, wild-type females administered 3.0 g/kg ethanol show significant hypolocomotion for the entire duration of the task, while PV:δ−/− begin to return to baseline activity sooner. d PV:δ−/− females also display dampened footslips following exposure to ethanol when compared to ethanol-treated wild-type females (*p < 0.05 compared to baseline; ^p < 0.05 compared to similarly treated wildtypes). e Wild-type males are significantly impaired following binge drinking (*p < 0.05 as compared to wildtypes) but PV:δ−/− males do not demonstrate similar motor incoordination f Though wild-type males display discrete minutes of hypolocomotion (*p < 0.05 as compared to saline), Area Under Curve Analysis (inset) show males do not display significant change in locomotor activity following this dose of ethanol g. PV:δ−/− males display dampened motor incoordination when compared to ethanol-treated wild-type males. (*p < 0.05, compared to baseline, ^p < 0.05, compared to similarly treated wildtypes)
Mice with parvalbumin-specific knockout of GABAA-δ are less sensitive to ethanol-induced motor incoordination and sedation and more sensitive to ethanol-associated hyperlocomotion
An alcohol naive cohort of PV:δ−/− mice and their WT counterparts were administered 1.75 g/kg ethanol (20%v/v; i.p.) and tested 10 min later on the balance beam. There was no effect of genotype on blood ethanol levels and mice displayed an ethanol content of 208.21 ± 32.37 mg/dL 10 min following drug administration. During the 10-min wait for balance beam testing, mice were placed in the open field and locomotor activity was recorded. Females showed a significant interaction of group and time on locomotor activity across the 10 min test [F(18,243) = 4.58, p < 0.0001]. Dunnett post hoc test confirmed that PV:δ−/− females maintained a more robust hyperlocomotor response to ethanol than wild-type mice, showing increased locomotion through the first 6 min of testing (p < 0.05) compared to the baseline activity of saline treated mice. Males also showed a significant interaction of group and time [F(18,252) = 2.997, p < 0.0001]. Post hoc test showed that PV:δ−/− males had a unique but transient hyperlocomotor response to ethanol (p < 0.05 for second minute) not seen for wild-type mice administered alcohol. Similarly, during the final minutes of testing, when activity for wild-type mice administered alcohol was significantly lower than baseline activity of saline treated mice (p < 0.05), PV:δ−/− males did not show this hypolocomotor response. To further evaluate this genotype-specific hypolocomotor response, a separate cohort of mice were included that received 0.5 g/kg and 3.0 g/kg in a random order design. There was an effect of genotype on hypolocomotion following 3.0 g/kg ethanol for females, though not for males. Specifically, PV:δ−/− females show a reduction in activity following just the first minute following ethanol at 3.0 g/kg (p < 0.05) while wild-type females demonstrate significant hypolocomotion throughout the 10 min test (p < 0.05). Both PV:δ−/− and wild-type males show significant hypolocomotion at 3.0 g/kg (p < 0.05). Neither males nor females of either genotype showed a treatment effect of 0.5 g/kg ethanol on activity in the open field (data not shown).
Following testing in the open field apparatus, mice were placed on the balance beam and footslips recorded. Females showed a significant effect of group [F(2,26) = 81.4, p < 0.0001; Fig. 4d]. Post hoc tests revealed that although this 1.75 g/kg administration of ethanol significantly increased footslips for both wildtype (M = 20.13 ± 1.90, n = 8) and PV:δ−/− females (M = 13.14 ± 1.61, n = 7) as compared to saline controls (M = 0.86 ± 0.27, n = 14; p < 0.0001), the level of intoxication for PV:δ−/− females was significantly lower than that of similarly treated wild-type mice (p < 0.01). Males also showed a significant effect of group [F(2,24) = 68.4, p < 0.0001; Fig. 4g], with both groups of mice administered ethanol showing significant intoxication when compared to saline treated controls (M = 1 ± 0.27, n = 11; p < 0.0001). However, this dose of ethanol resulted in significantly fewer footslips in PV:δ−/− males (M = 13 ± 1.4, n = 9) as compared to the wild-type males administered the same dose of ethanol (M = 18.29 ± 1.57, n = 7; p < 0.05).
Experiment 3: Do δ-GABAARs on PV-positive interneurons play a role in the behavioral consequences of binge drinking?
Mice with parvalbumin-specific knockout of GABAA-δ do not display anxiety-like behaviors after a history of heavy binge drinking
Following a 2-week history of binge drinking, mice were given 1 day of forced abstinence before assessing anxiety-like behaviors using the light dark emergence task. Time in the dark, distance traveled in the dark, percent of activity in the dark (distance traveled in the dark/total distance) and the time (s) to first emerge from the dark side of the light/dark box were used as indices anxiety-like behavior.
There was a significant effect of group on preference for the dark [F(3,40) = 3.44, p < 0.05; Fig. 5]. However, Bonferroni adjusted multiple comparisons showed that wild-type females (M = 79.84% ± 6.33) did not display increased preference for the dark when compared to their water drinking controls (M = 74.34% ± 3.83) during this early withdrawal period. Instead, group differences were driven by PV:δ−/− females with a history of binge drinking (M = 59% ± 4.08) who showed significantly lower preference for the dark side than wild-type mice with a similar history (p < 0.05). There was a marginal effect of group on the time spent in the dark during the task [F(3,40) = 2.54, p = 0.06; Fig. 5b], with wild-type binge drinkers (M = 239.2 ± 20.38) spending similar time in dark as wild-type water drinkers (M = 226.3 ± 11.22) and PV:δ−/− water drinkers but 20% more time in the dark than similarly treated PV:δ−/− females (M = 186.6 ± 11.33). There was a significant effect of group on emergence delay [F(3,42) = 7.14, p < 0.0001; Fig. 5e]. In particular, wild-type females with a history of binge drinking (M = 158 ± 39.65) took almost three times as long as their water drinking controls (M = 55.92 ± 14.59) to emerge from the dark side of the box (p < 0.01), supporting anxiety-like behavior during withdrawal. PV:δ−/− females who consumed alcohol (M = 34.36 ± 18.04) took a similar amount of time to emerge from the dark and explore the anxiogenic side of the box as their water drinking counterparts (M = 21.8 ± 7.37). As with dark side activity, wild-type females with a history of binge drinking took significantly longer than similarly treated PV:δ−/− females to emerge from the dark side of the box than wild-type females (p < 0.001).
PV:δ−/− mice do not display anxiety-like behavior during withdrawal. a Mice had access to ethanol (or water) using DID-MSA for 14 days and were tested following 1 day of forced abstinence in the light dark box for 10 min. Brains were collected and trunk bloods sampled 24 h after behavioral testing. b There was no effect on time in the dark side of the chamber in any group for female mice. c Female wild-type mice do not display change in exploration of the dark box during early withdrawal, though PV:δ−/− females have reduced dark box exploration (reduced anxiety) when compared to similarly treated wild-type females. d Females did not demonstrate a relationship between corticosterone and average ethanol consumed during the 2 week access period. e Wild-type females show significant increase in time it takes to emerge into the light side of the box (more anxiety-like behavior) after 2 weeks of binge drinking whereas PV:δ−/− females do not. f Though there was no group effect on time in the dark, wild-type males do show a marginal increase in time spent in the dark side of the box following binge drinking (p = 0.06). g Only wild-type males show increased exploration of the dark box (increased anxiety-like behavior) following 2 weeks of binge drinking. h Males show significant relationship between circulating corticosterone during early withdrawal and the average amount of daily alcohol consumed during DID-MSA. i Emergence delay was not altered by alcohol exposure. (*p < 0.05, compared to baseline, ^p < 0.05, compared to similarly treated wildtypes)
There was a significant effect of group on preference for the dark side of the box for males [F(3,26) = 3.9, p < 0.02; Fig. 5]. Bonferroni adjusted comparisons confirmed that wild-type males with a history of binge drinking displayed significantly more preference for the dark side of the box (p < 0.05; M = 65.19% ± 2.34; n = 6) than their water drinking controls (M = 52.68% ± 1.86; n = 9), while PV:δ−/− males who consumed ethanol displayed similar dark side activity preference (M = 60.47% ± 3; n = 6) as PV:δ−/− males who consumed water (M = 58.41% ± 3.63; n = 7). Unlike females, males did not show a significant group effect on time to emerge from the dark side of the box (p = 0.16; n.s.) nor an effect of group on time in the dark (p = 0.81; n.s.).
Discussion
The data presented herein support a significant role for δ containing GABAA receptors (δ-GABAARs) in interneurons, specifically PV-positive interneurons, in both the maintenance of binge alcohol drinking and in the maladaptive development of anxiety that follows this pattern of alcohol use. We demonstrate for the first time that voluntary binge drinking associated with the development of anxiety-like behavior during early withdrawal causes an interneuron restricted increase in the expression of the GABAAR δ subunit in female mice. Our findings also show that the restricted deletion of δ-GABAARs from PV-positive interneurons results in an enhanced heavy binge drinking phenotype in males, alters response to the effects of ethanol on locomotor behavior and motor coordination in both males and females. Deletion of the GABAAR δ subunit from PV-positive interneurons also protects mice from the development of anxiety-like behavior during withdrawal from heavy drinking in both sexes. Together, these data strongly support δ-GABAARs in PV interneurons as an integral target for binge alcohol drinking and its behavioral consequences.
Evidence supporting δ-GABAARs as a target for alcohol
Although alcohol has not been shown to directly interact with δ-GABAARs, a growing body of evidence shows that the drug can augment tonic inhibition in a number of systems. Experiments using hippocampal slice preparations confirmed that this increase in tonic inhibition is mediated by extrasynaptic δ containing GABAA receptors (δ-GABAAR), as it may be seen in the dentate gyrus granule cells, but not in CA1 pyramidal cells, where tonic inhibition is mediated by α5 containing extrasynaptic GABAA receptors that do not express the δ subunit [16]. Relatedly, acute alcohol may increase synthesis and translocation of steroidogenic acute regulatory protein [44,45,46], an integral protein in the rate-limiting step in the steroid synthesis pathway. This may underlie the increase in synthesis and availability of neurosteroids following acute ethanol ([47]; [22, 23, 48,49,50]). With this, acute ethanol may augment δ-mediated tonic inhibition and that chronic ethanol exposure may lead to long-term adaptations in this system. Indeed, adult rats chronically exposed to ethanol show reduced expression of δ protein in the hippocampus [12, 51] and BLA [13]. Whether these latter changes are relevant to the effects of ethanol seen in non-dependent binge drinkers is unclear, as these studies used significant exposure paradigms. On the other hand, studies modeling binge drinking have all failed to support changes in this GABAA receptor protein in adult exposed rats. In these studies, only adolescents exposed to ethanol show long-lasting downregulation in δ protein in the hippocampus and correspondingly, downregulation of δ-mediated tonic inhibition in this region [25, 26]. In this latter case, shorter term ethanol exposure also increases the ethanol-induced enhancement of δ-mediated tonic conductance in dentate gyrus granule cells. The focus solely on principal neurons may overlook the potential role that interneurons may play in the effects of alcohol. In support of this, we find that voluntary binge drinking in mice leads to significant increases in δ protein expression specific to parvalbumin interneurons in the hippocampus and basolateral amygdala, where no changes were found using a broader region-based assessment. Thus, we demonstrate that short term but heavy binge drinking in adults leads to both anxiety-like behavior and changes in δ expression on interneurons.
The development of the global δ knockout mouse was instrumental in our understanding of the significant role that δ-GABAAR mediated tonic inhibition may play in the effects of alcohol. These mice displayed lower sensitivity to the handling-induced convulsions associated with ethanol withdrawal and had lower sensitivity to ethanol-induced motor incoordination [52]. Though this reduced sensitivity would suggest a greater capacity for the consumption of ethanol, male δ−/− mice display lower intake of alcohol in a 24 h preference paradigm, while females show modest reductions in their ethanol preference behavior [52]. Since then, a number of pharmacological studies have confirmed that δ-GABAARs are a relevant target for alcohol consumption, as activating this receptor population with THIP prior to drinking reduces ethanol intake behaviors in mice [18,19,20,21]. Interestingly, genetic ablation of this receptor system-either locally in regions like the nucleus accumbens [17] or globally as in the case of δ−/− knockout mice [52], also reduces alcohol drinking. This first appears to be a contradiction in the effects of pharmacological activation and genetic ablation of the same receptor subtype. However, when one considers that δ-GABAARs may be expressed by both excitatory cells and by interneurons that regulate these excitatory cells, it is clear that the cellular subtype matters significantly to the overall effect that modulation of δ-GABAARs may have on a given behavior. Indeed, our PV-specific deletion of δ, unlike global deletion of this GABAAR subunit, is associated with an increase in intake. Interestingly, similar to previous findings, our data suggests that the effects of δ manipulation on intake may be sex specific. Global deletion of δ was associated with a greater reduction in alcohol preference for males than females [52]. Our results here demonstrate that PV-specific deletion of δ produces a male-specific increase in binge drinking. Recent work supporting sex differences in the expression of δ mRNA and protein show lower δ mRNA in females [53]. Relatedly, studies on δ-GABAA regulation across the estrous cycle in females show that δ-mediated tonic current [54] and δ mRNA and protein [20, 53,54,55,56] are all lower in the estrus phase as compared to the diestrus phase. Interestingly δ-mediated tonic current during this diestrus phase-when δ expression is high- is more similar to that seen in males than the other phases [54]. Together, these findings suggest that the higher δ expression in males could play a role in noted sex differences in ethanol intake [57]. However, it is unclear whether the male-specific increase in intake following deletion of δ in PV interneurons noted here truly is related to biological sex, or a product of a ceiling effect; as females in the DID-MSA limited access paradigm consume significantly more ethanol than males, there may be little room to see greater intake using short periods of ethanol access. Future studies clarifying the role that δ-GABAA receptors may play in sex differences in ethanol preference and voluntary consumption are warranted.
Ethanol has a biphasic effect on locomotion, with lower blood ethanol concentrations causing an initial stimulant response and higher concentrations producing hypolocomotion or sedation. Our data suggest a role for δ-GABAARs in sensitivity to the stimulant side of this biphasic response curve, given that the change in locomotor activity following ethanol is greater for female PV:δ−/−. We do not see this for males, although their stimulant response appears to be reduced during this period than females, as others have found [58]. Evidence supports a positive relationship between the stimulant response to alcohol during the rising phase of the alcohol concentration curve and future binge drinking [59], progression of alcohol use disorder [60, 61] and current heavy drinking status [62]. Future work should clarify a potential role for interneuronal δ-GABAARs in ethanol-induced motor incoordination and/or sedation. Whether changes in δ expression demonstrated at 48 h following cessation of binge drinking in wild-type mice are relevant to their behavioral dysfunction measured at 24–32 h following cessation of binge drinking and the role that these differences in the acute reactivity to the drug across these genotypes warrants further investigation.
Maladaptive behaviors following binge drinking
Much of the concern regarding the increasing prevalence of binge drinking comes from the relationship between this pattern of alcohol use and the development of an alcohol use disorder (AUD), with about 10% of binge drinkers having an AUD [2]. However, studies showing mood disturbances and anxiety in non-dependent binge drinkers suggest other psychiatric disorders may precede the development of an AUD in this population and proper treatment associated with these affective dysfunctions may offer an opportunity to block the development of further problem drinking.
Extended binge drinking may produce anxiety-like behavior [6]. Previous studies demonstrate that acute alcohol may reduce the time rodents take to emerge from the dark to the light [63]. Here we demonstrate anxiety-like behavior during early withdrawal from binge drinking and that this maladaptation was blocked in PV:δ−/− mice. Together, these data support δ on PV- interneurons as a significant mechanism driving anxiety-like behavior following binge drinking. Though this also suggests a potential role for δ-GABAARs on parvalbumin interneurons in mediating the anxiolytic effects of acute alcohol, this remains to be explored.
Together, the data presented herein demonstrate that δ-GABAARs in PV-positive interneurons are not only a relevant target for the long-term effects of binge drinking, but may regulate the behavior itself, potentially via its role in mediating the sedative effects of alcohol or the rewarding/stimulating effects of the compound. Identifying molecular differences in this subtype of δ-GABAARs vs δ-GABAARs expressed by other cell types offers a potential therapeutic target for the reversal of anxiety associated with heavy drinking.
References
Organization WH (2014). Global status report on alcohol and health, 2014 World Health Organization.
Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among US adult drinkers, 2009-2011. Prev Chronic Dis. 2014;11:1–11.
Paljärvi T, Koskenvuo M, Poikolainen K, Kauhanen J, Sillanmäki L, Mäkelä P. Binge drinking and depressive symptoms: a 5‐year population‐based cohort study. Addiction. 2009;104:1168–78.
Townshend JM, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol: Clin Exp Res. 2005;29:317–25.
Lee K, Coelho M, Sern K, Szumlinski K. Homer2 within the central nucleus of the amygdala modulates withdrawal-induced anxiety in a mouse model of binge-drinking. Neuropharmacology. 2018;128:448–59.
Lee KM, Coehlo M, McGregor HA, Waltermire RS, Szumlinski KK. Binge alcohol drinking elicits persistent negative affect in mice. Behav Brain Res. 2015;291:385–98.
Stevenson JR, Schroeder JP, Nixon K, Besheer J, Crews FT, Hodge CW. Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology. 2009;34:1209–22.
Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. GABA transport modulates the ethanol sensitivity of tonic inhibition in the rat dentate gyrus. Alcohol. 2011;45:577–83.
Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol. 2007;97:3806–11.
Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–8.
Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8:339–45.
Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–58.
Lindemeyer AK, Liang J, Marty VN, Meyer EM, Suryanarayanan A, Olsen RW, et al. Ethanol-induced plasticity of GABAA receptors in the basolateral amygdala. Neurochem Res. 2014;39:1162–70.
Centanni SW, Burnett EJ, Trantham-Davidson H, Chandler LJ. Loss of delta-GABAA receptor-mediated tonic currents in the adult prelimbic cortex following adolescent alcohol exposure. Addict Biol. 2017;22:616–28.
Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–23.
Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–82.
Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic delta-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci USA. 2011;108:4459–64.
Ramaker MJ, Ford MM, Fretwell AM, Finn DA. Alteration of ethanol drinking in mice via modulation of the GABA(A) receptor with ganaxolone, finasteride, and gaboxadol. Alcohol Clin Exp Res. 2011;35:1994–2007.
Fritz BM, Boehm SL 2nd. Site-specific microinjection of Gaboxadol into the infralimbic cortex modulates ethanol intake in male C57BL/6J mice. Behav Brain Res. 2014;273:8–15.
Melón LC, Nolan ZT, Colar D, Moore EM, Boehm SL 2nd. Activation of extrasynaptic delta-GABAA receptors globally or within the posterior-VTA has estrous-dependent effects on consumption of alcohol and estrous-independent effects on locomotion. Horm Behav. 2017;95:65–75.
Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL 2nd. GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–13.
Finn DA, Jimenez VA. Dynamic adaptation in neurosteroid networks in response to alcohol. Handb Exp Pharmacol. 2017.
Porcu P, Morrow AL. Divergent neuroactive steroid responses to stress and ethanol in rat and mouse strains: relevance for human studies. Psychopharmacology. 2014;231:3257–72.
Cook JB, Werner DF, Maldonado-Devincci AM, Leonard MN, Fisher KR, O’Buckley TK, et al. Overexpression of the steroidogenic enzyme cytochrome P450 side chain cleavage in the ventral tegmental area increases 3alpha,5alpha-THP and reduces long-term operant ethanol self-administration. J Neurosci. 2014b;34:5824–34.
Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcohol Clin Exp Res. 2012;36:279–85.
Fleming RL, Li Q, Risher ML, Sexton HG, Moore SD, Wilson WA, et al. Binge-pattern ethanol exposure during adolescence, but not adulthood, causes persistent changes in GABAA receptor-mediated tonic inhibition in dentate granule cells. Alcohol Clin Exp Res. 2013;37:1154–60.
Liang J, Shen Y, Shao XM, Scott MB, Ly E, Wong S, et al. Dihydromyricetin prevents fetal alcohol exposure-induced behavioral and physiological deficits: the roles of GABAA receptors in adolescence. Neurochem Res. 2014b;39:1147–61.
Liang J, Lindemeyer AK, Suryanarayanan A, Meyer EM, Marty VN, Ahmad SO, et al. Plasticity of GABA(A) receptor-mediated neurotransmission in the nucleus accumbens of alcohol-dependent rats. J Neurophysiol. 2014a;112:39–50.
Gatta E, Auta J, Gavin DP, Bhaumik DK, Grayson DR, Pandey SC, et al. Emerging role of one-carbon metabolism and DNA methylation enrichment on delta-containing GABAA receptor expression in the cerebellum of subjects with alcohol use disorders (AUD). Int J Neuropsychopharmacol. 2017;20:1013–26.
Shen Y, Lindemeyer AK, Spigelman I, Sieghart W, Olsen RW, Liang J. Plasticity of GABAA receptors after ethanol pre-exposure in cultured hippocampal neurons. Mol Pharmacol. 2011;79:432–42.
McDonald AJ, Mascagni F. Parvalbumin-containing interneurons in the basolateral amygdala express high levels of the alpha1 subunit of the GABAA receptor. J Comp Neurol. 2004;473:137–46.
Hale MW, Johnson PL, Westerman AM, Abrams JK, Shekhar A, Lowry CA. Multiple anxiogenic drugs recruit a parvalbumin-containing subpopulation of GABAergic interneurons in the basolateral amygdala. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1285–93.
Herman MA, Contet C, Justice NJ, Vale W, Roberto M. Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci. 2013;33:3284–98.
Herman MA, Roberto M. Cell-type-specific tonic GABA signaling in the rat central amygdala is selectively altered by acute and chronic ethanol. Addict Biol. 2016;21:72–86.
Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–45.
Lee V, Sarkar J, Maguire J. Loss of Gabrd in CRH neurons blunts the corticosterone response to stress and diminishes stress-related behaviors. Psychoneuroendocrinology. 2014;41:75–88.
Ferando I, Mody I. Altered gamma oscillations during pregnancy through loss of delta subunit-containing GABA(A) receptors on parvalbumin interneurons. Front Neural Circuits. 2013;7:144.
Ferando I, Mody I. In vitro gamma oscillations following partial and complete ablation of delta subunit-containing GABAA receptors from parvalbumin interneurons. Neuropharmacology. 2015;88:91–8.
Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, McBride WJ. Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol Biochem Behav. 2011;100:90–7.
Melon LC, Wray KN, Moore EM, Boehm SL 2nd. Sex and age differences in heavy binge drinking and its effects on alcohol responsivity following abstinence. Pharmacol Biochem Behav. 2013;104:177–87.
Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci. 2011;31:18198–210.
Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci. 2009;29:9592–601.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Khisti RT, Kumar S, Morrow AL. Ethanol rapidly induces steroidogenic acute regulatory protein expression and translocation in rat adrenal gland. Eur J Pharmacol. 2003;473:225–7.
Kim HJ, Ha M, Park CH, Park SJ, Youn SM, Kang SS, et al. StAR and steroidogenic enzyme transcriptional regulation in the rat brain: effects of acute alcohol administration. Brain Res Mol Brain Res. 2003;115:39–49.
Serra M, Pisu MG, Floris I, Cara V, Purdy RH, Biggio G. Social isolation-induced increase in the sensitivity of rats to the steroidogenic effect of ethanol. J Neurochem. 2003;85:257–63.
Cook JB, Dumitru AM, O’Buckley TK, Morrow AL. Ethanol administration produces divergent changes in GABAergic neuroactive steroid immunohistochemistry in the rat brain. Alcohol Clin Exp Res. 2014a;38:90–9.
Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, et al. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23:1933–40.
Porcu P, O’Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, et al. Differential effects of ethanol on serum GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in mice, rats, cynomolgus monkeys, and humans. Alcohol Clin Exp Res. 2010;34:432–42.
Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, et al. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–30.
Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64.
Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, et al. GABA(A)-receptor delta subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25:1708–18.
Tonsfeldt KJ, Suchland KL, Beeson KA, Lowe JD, Li MH, Ingram SL. Sex differences in GABAA signaling in the periaqueductal gray induced by persistent inflammation. J Neurosci. 2016;36:1669–81.
Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804.
Lovick TA, Griffiths JL, Dunn SM, Martin IL. Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405.
Wu X, Wu Z, Ning G, Guo Y, Ali R, Macdonald RL, et al. gamma-Aminobutyric acid type A (GABAA) receptor alpha subunits play a direct role in synaptic versus extrasynaptic targeting. J Biol Chem. 2012;287:27417–30.
Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharmacol Rev. 2016;68:242–63.
Quadir SG, Guzelian E, Palmer MA, Martin DL, Kim J, Szumlinski KK. Complex interactions between the subject factors of biological sex and prior histories of binge-drinking and unpredictable stress influence behavioral sensitivity to alcohol and alcohol intake. Physiol Behav. 2017:1–24.
King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–99.
King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao D. A prospective 5-Year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol Psychiatry. 2016;79:489–98.
King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry. 2014;75:798–806.
Roche DJ, Palmeri MD, King AC. Acute alcohol response phenotype in heavy social drinkers is robust and reproducible. Alcohol Clin Exp Res. 2014;38:844–52.
Sharko AC, Kaigler KF, Fadel JR, Wilson MA. Ethanol-induced anxiolysis and neuronal activation in the amygdala and bed nucleus of the stria terminalis. Alcohol. 2016;50:19–25.
Funding
J.M. is supported by the NIH-NINDS grant R01 NS073574 (J.M.). L.C.M. is supported by the NIH-NIGMS grant K12GM074869; an IRACDA postdoctoral training grant to Tufts University (Training in Education and Critical Research Skills: TEACRS). J.T.N., A.St.J. and K.M. were supported by the Tufts University’s Building Diversity in Biomedical Research program. The behavioral and imaging studies were conducted in the Tufts Center for Neuroscience Research, P30 NS047243. J.M. serves on the Scientific Advisory Board for SAGE Therapeutics and receives financial support for research that is unrelated to the current study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
LCM, JTN, ASJ, and KM report no competing financial interests or conflicts of interest. JLM is a member of the Scientific Advisory Board and consultant for SAGE Therapeutics, a relationship overseen by Tufts University and does not represent a conflict with the current study.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Melón, L.C., Nasman, J.T., John, A.S. et al. Interneuronal δ-GABAA receptors regulate binge drinking and are necessary for the behavioral effects of early withdrawal. Neuropsychopharmacol 44, 425–434 (2019). https://doi.org/10.1038/s41386-018-0164-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-018-0164-z