Abstract
Persistent susceptibility to cue-induced relapse is a cardinal feature of addiction. Discriminative stimuli (DSs) are one type of drug-associated cue that signal drug availability (DS+) or unavailability (DS−) and control drug seeking prior to relapse. We previously established a trial-based procedure in rats to isolate DSs from context, conditioned stimuli, and other drug-associated cues during cocaine self-administration and demonstrated DS-controlled cocaine seeking up to 300 abstinence days. The behavioral and neural mechanisms underlying trial-based DS-control of drug seeking have rarely been investigated. Here we show that following discrimination training in our trial-based procedure, the DS+ and DS− independently control the expression and suppression of cocaine seeking during abstinence. Using microinjections of GABAA + GABAB receptor agonists (muscimol + baclofen) in medial prefrontal cortex, we report that infralimbic, but not prelimbic, subregion of medial prefrontal cortex is critical to persistent DS-controlled relapse to cocaine seeking after prolonged abstinence, but not DS-guided discriminated cocaine seeking or DS-controlled cocaine self-admininstration. Finally, using ex vivo whole-cell recordings from pyramidal neurons in the medial prefrontal cortex, we demonstrate that the disruption of DS-controlled cocaine seeking following infralimbic cortex microinjections of muscimol+baclofen is likely a result of suppression of synaptic transmission in the region via a presynaptic mechanism of action.
Similar content being viewed by others
Introduction
Relapse is a cardinal feature of addiction [1, 2]. In both human and rodent models, environmental stimuli previously paired with drug self-administration can elicit drug seeking even after prolonged abstinence [3,4,5,6,7,8,9]. Discriminative stimuli (DSs) are one type of drug-associated cue that can signal either drug availability (DS+) or unavailability (DS−) to guide drug seeking and taking [7]. They play important roles in relapse because they precede and guide drug seeking prior to drug taking and are difficult to extinguish [7, 10,11,12,13,14,15,16,17,18,19,20,21].
In most studies of DS-controlled drug seeking, the DS+ and DS− were presented in separate sessions and investigators used procedures that did not clearly isolate their effects from other drug-associated stimuli, such as context or discrete conditioned stimuli (CSs) [7, 10,11,12,13, 16,17,18,19, 22]. In contrast, trial-based procedures for studying DS-control allow isolation of DSs from other stimuli [14, 20, 23, 24] to identify their unique contribution to drug-seeking behavior. Incorporation of many, intermixed, repeated trials also enables investigation of DS-specific neuronal ensemble activity using in vivo electrophysiology or calcium imaging [25,26,27,28,29,30,31]. Based on these considerations, we developed a trial-based procedure in rats that used DS+ and DS− with a common lever manipulandum and no drug infusion-paired cues [21, 32], to isolate the unique effects of the DSs beyond that of previous studies [14, 23, 24]. Following trial-based discrimination, we assessed DS-control of cocaine seeking during abstinence and showed persistent non-reinforced drug seeking during DS+ (but not DS−) presentations, up to 300 days after the last DS-drug pairing [21].
The neural mechanisms of trial-based DS control of drug [14, 20, 23, 24] or non-drug [25,26,27,28,29,30,31] reward seeking have not often been studied. The medial prefrontal cortex (mPFC) has been implicated in drug and non-drug reward seeking [33,34,35,36,37,38]. Early studies using the extinction-reinstatement model with cocaine suggested a functional dichotomy wherein prelimbic cortex (PL) activity promotes cocaine seeking while infralimbic cortex (IL) activity suppresses cocaine seeking [39,40,41]. However, recent studies have shown opposite and sometimes overlapping roles for these subregions in reward seeking, depending on the reinforcer type [42,43,44,45,46,47], abstinence-induced manipulation (forced abstinence versus extinction) [46, 48,49,50], the stimulus used to induce reward seeking [13, 18, 51,52,53,54,55], or the neural manipulation (global inactivation versus Daun02 selective inactivation of Fos-expressing ensemble neurons) [56, 57]. While some studies showed that prior extinction training is necessary for IL inhibitory control [58, 59] other studies suggest extinction-independent roles for PL and IL in drug seeking during abstinence [48, 60, 61]. However, these studies mainly focused on how mPFC activity guides drug seeking in response to contextual or drug-paired stimuli, or drug priming;[9, 62] only a few examined the role of PL and IL in DS-controlled drug and non-drug reward seeking and taking [13, 18, 20, 27, 28, 52, 53].
We first investigated individual contributions of DS+ and DS− to persistent cocaine seeking after 21 abstinence days by measuring non-reinforced lever-presses during 4 trial types: no-DSs, DS+, DS−, and both-DSs. Next, we used microinjections of GABAA + GABAB receptor agonists (muscimol + baclofen, M + B) to examine the role of PL and IL in DS-controlled relapse to cocaine seeking after 21 abstinence days and in ongoing DS-controlled cocaine self-administration. Finally, we used whole-cell voltage clamp recordings in an ex vivo brain slice preparation to identify candidate synaptic mechanisms underlying M + B effects that might contribute to DS-control of relapse to cocaine seeking.
Materials and methods
A detailed description of experimental subjects, apparatus, and procedures is included as Supplementary Online Methods. All procedures were approved by the NIDA IRP Animal Care and Use Committee and followed guidelines outlined in the Guide for the Care and Use of Laboratory Animals [63]. Below we provide an overview of the experiments.
Individual contributions of DS+ and DS− to DS-controlled cocaine seeking during abstinence (Experiment 1)
The goal of this experiment was to determine how the two DSs used in our procedure [21] exert stimulus control over cocaine seeking during abstinence. We first trained male rats to either lever press for cocaine (0.75 mg/kg/infusion) when a light cue (DS+) signaled cocaine availability (DS+ trials) or suppress lever-pressing when a different light cue (DS−) signaled cocaine unavailability (DS− trials) during the same session; DS+/− trials were presented in pseudorandom order. After training, we tested the rats for DS-controlled cocaine seeking on day 1 and placed them in their homecage for 20 days of forced abstinence. We then investigated the individual contributions of DS+ and DS− to discriminated cocaine seeking on abstinence day 21. We measured non-reinforced responses during each of 4 possible combinations of DS+ (on, off) and DS- (on, off) trial types: no-DSs, DS+, DS−, or both-DSs (15 presentations per type; pseudorandom order).
Role of IL (Experiment 2) and PL (Experiment 3) neural activity in DS-controlled cocaine seeking
The goal of these experiments was to examine the role of IL and PL activity in discriminated cocaine seeking. Following training and a period of forced abstinence, we tested whether M + B microinjections (0.03 nM muscimol + 0.3 nM baclofen per side; 0.5 µL injection) into IL or PL would affect DS-controlled cocaine seeking on abstinence day 21.
Role of IL and PL neural activity in DS-controlled cocaine self-administration (Experiment 4)
The goal of this experiment was to examine the role of IL or PL activity in ongoing DS-controlled cocaine self-administration. Following training, we tested whether M + B microinjections into IL or PL would affect ongoing discriminated cocaine self-administration. We retrained rats between tests and tested those that showed stable discriminated drug-taking behavior.
Effect of pharmacological manipulation of GABA receptors in IL neurons using ex vivo brain slice electrophysiology (Experiment 5)
The goal of this experiment was to determine the effect of M + B on synaptic activity using whole cell voltage-clamp recordings (Vhold = −70 mV) in visually identified layer 5/6 pyramidal neurons within IL. We recorded spontaneous synaptic responses and then used electrical stimulation to evoke synaptic responses. After obtaining a stable baseline recording of both evoked and spontaneous excitatory postsynaptic currents (EPSCs), we bath-applied M + B for 10–15 min and determined the percent change from baseline after drug application.
Results
Discrimination training
Discrimination training was performed identically for experiments 1–4 (see Figs. 1–4, and Fig. S1). Rats learned to lever press for cocaine infusions (left graph panel B), continued responding during trial training (center graph panel B) and then learned to discriminate DS+ from DS− during discrimination training (right graph panel B). There were no group differences in acquisition of discrimination training or during DS-controlled cocaine seeking on day 1 for rats subsequently tested under the different experimental conditions. See supplementary tables S1–6 for a detailed listing of experimental subjects and statistical analyses.
A Experimental timeline. B Training data. Self-administration: Rats learned to self-administer cocaine over 6 sessions. Mean (±SEM) number of cocaine infusions and lever presses during each 3-h session. Trial training: Mean (±SEM) number of lever presses and infusions received during the 3-h sessions (30 DS+ trials in the AM session, 30 DS− trials in the PM session). Discrimination training: All rats learned to discriminate DS+ from DS− trials within the same session (30 trials each of DS+ and DS− trials presented in a pseudorandomized manner). Mean (±SEM) number of lever presses during each trial type (top), and infusions received (bottom) during a 3-h discrimination training session. * indicates significant difference (p < 0.05) between responding during DS+ and DS− trial types (n = 27). C: Relapse tests. Day 1: Rats showed reliable DS-controlled cocaine seeking on abstinence day 1 when presented with 30 trials each of DS+ and DS− (pseudorandomized order, extinction conditions). Columns indicate Mean (±SEM) number of trials and lever presses during the 3-h day 1 relapse test session separated by 2 trial types, while dots indicate values for individual rats. * denotes significant difference (p < 0.05) in responding between DS+ and DS− trials. Day 21 (Cue test): Rats maintained DS-controlled cocaine seeking on abstinence day 21 when presented with 15 each of no-DSs, DS+, DS−, and both-DSs trials (pseudorandomized order, extinction conditions). Non-reinforced responding was low during no-DSs and DS- trials, intermediate during both-DSs trials, and maximal during DS+ trials indicating that following discrimination training in this task, the DS+ and DS− independently control the expression and suppression of DS-controlled drug seeking during abstinence. Columns indicate Mean (±SEM) number of trials and lever presses during the 3-h day 21 relapse test session separated by 4 trial types, while dots indicate values for individual rats. * denotes significant difference (p < 0.05) in responding between trial types indicated by the solid line (n = 27).
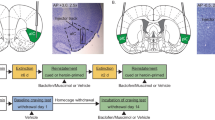
A Experimental timeline. B Training data. Self-administration: Rats learned to self-administer cocaine over 6 sessions. Mean (±SEM) number of cocaine infusions and lever presses during each 3-h session. Trial training: Mean (±SEM) number of lever presses and infusions received during the 3-h sessions (30 DS+ trials in the AM session, 30 DS− trials in the PM session). Discrimination training: All rats learned to discriminate DS+ from DS− trials within the same session (30 trials each of DS+ and DS− trials presented in a pseudorandomized manner). Mean (±SEM) number of lever presses during each trial type (top), and infusions received (bottom) during a 3-h discrimination training session. * indicates significant difference (p < 0.05) between responding during DS+ and DS− trial types (n = 35). C Relapse tests. There were no group differences in DS-controlled cocaine seeking after 1 day of abstinence (left panel, no microinjections). Microinjections of M + B into IL significantly reduced cocaine seeking during both DS+ and DS− trials following 21 days of abstinence as compared to vehicle-injected controls. Mean (±SEM) number of lever presses during the entire 3-h relapse test session (day 1—left panel; day 21—right panel). * indicates significant difference (p < 0.05) in responding during DS trials between vehicle and M + B groups. # indicates significant difference (p < 0.05) between responding during DS+ and DS− trial types within a treatment group (n = 17 for vehicle; n = 18 for M + B). D Cannula placements. Placement of injector tips was determined using cresyl violet counterstaining of formalin-fixed tissue.
A Experimental timeline. B Training data. Self-administration: Rats learned to self-administer cocaine over 6 sessions. Mean (±SEM) number of cocaine infusions and lever presses during each 3-h session. Trial training: Mean (±SEM) number of lever presses and infusions received during the 3-h sessions (30 DS+ trials in the AM session, 30 DS− trials in the PM session). Discrimination training: All rats learned to discriminate DS+ from DS− trials within the same session (30 trials each of DS+ and DS− trials presented in a pseudorandomized manner). Mean (±SEM) number of lever presses during each trial type (top), and infusions received (bottom) during a 3-h discrimination training session. * indicates significant difference (p < 0.05) between responding during DS+ and DS− trial types (n = 30). C Relapse tests. There were no group differences in DS-controlled cocaine seeking after 1 day of abstinence (left panel, no microinjections). Microinjections of M + B into PL did not disrupt DS-controlled cocaine seeking following 21 days of abstinence as compared to vehicle-injected controls. Mean (±SEM) number of lever presses during the entire 3-h relapse test session (day 1—left panel; day 21—right panel). # indicates significant difference (p < 0.05) between responding during DS+ and DS− trial types within a treatment group (n = 15 for vehicle; n = 15 for M + B). D Cannula placements. Placement of injector tips was determined using cresyl violet counterstaining of formalin-fixed tissue.
A Experimental timeline. B Training data. Self-administration: Rats learned to self-administer cocaine over 8 sessions. Mean (±SEM) number of cocaine infusions and lever presses during each 3-h session. Trial training: Mean (±SEM) number of lever presses and infusions received during the 3-h sessions (30 DS+ trials in the AM session, 30 DS- trials in the PM session). Discrimination training: All rats learned to discriminate DS+ from DS− trials within the same session (30 trials each of DS+ and DS− trials presented in a pseudorandomized manner). Mean (±SEM) number of lever presses during each trial type (top), and infusions received (bottom) during a 3-h discrimination training session. * indicates significant difference (p < 0.05) between responding during DS+ and DS− trial types (n = 14, all tested rats). C Discrimination tests following microinjections. M + B (counterbalanced with corresponding vehicle using a within-subjects design, 2 tests/region) was microinjected into either IL or PL prior to a discriminated cocaine self-administration session. Rats were retrained between tests and testing was restricted to rats that maintained discriminated drug-taking behavior. Microinjections of M + B into either PL (bottom left, n = 11) or IL (bottom right, n = 12) did not disrupt DS-controlled cocaine taking. Mean (±SEM) number of lever presses during each trial type during 3-h DS-controlled cocaine self-administration sessions following microinjections into IL or PL. # indicates significant difference (p < 0.05) between responding during DS+ and DS− trial types within a treatment group. D Cannula placements. Placement of injector tips was determined using cresyl violet counterstaining after the last test (n = 14, all tested rats). PL injection locations were 2.2 mm dorsal to those shown in the figure.
Experiment 1: Individual contributions of DS+ and DS− to persistent DS-controlled cocaine seeking
Following discrimination training, we first tested rats for discriminated cocaine seeking on abstinence day 1. The number of trials with at least one lever press (denoted as trials) and total number of lever presses (denoted as lever presses) were recorded separately for each DS trial type during each session and analyzed using the within-subject factor of DS (DS+, DS−). Rats responded on more DS+ trials than DS− trials (t26 = 6.7, p < 0.0001); they also made more lever presses during DS+ trials (t26 = 6.5, p < 0.0001), indicating that cocaine seeking was under DS control (Fig. 1C, left panel). We then placed rats in their homecage for 20 days and tested for DS-controlled cocaine seeking on abstinence day 21 using a modified version of the day 1 seeking test with 4 trial conditions: no-DSs, DS+, DS−, or both-DSs. We analyzed both trials and lever presses measures using two-way ANOVAs with within-subject factors of DS+ (on, off) and DS− (on, off) and observed significant interaction between the two factors (trials: F1,26 = 10.4, p = 0.0033; lever presses: F1,26 = 12.7, p = 0.0014). For both measures, responding was low during ‘no-DSs’ and ‘DS−’ trials, intermediate during ‘both-DSs’ trials, and maximal during ‘DS+’ trials (see Table S2 for full statistical results). These results indicate that DS+ and DS− independently control the expression (i.e., increased responding during ‘DS+’ relative to ‘no-DSs’ trials) and suppression (i.e., decreased responding during ‘both-DSs’ relative to ‘DS+’ trials) of cocaine seeking during abstinence.
Experiment 2: Microinjections of M + B into IL prior to DS-controlled cocaine seeking
Following discrimination training, we first tested rats for cocaine seeking on abstinence day 1 (Fig. 2C, left panel). We then placed the rats in their homecage for 20 days and tested whether M + B microinjections into IL would affect DS-controlled cocaine seeking on abstinence day 21. We analyzed the number of non-reinforced lever presses during the test session using mixed ANOVA with within-subject factor of DS (DS+, DS−) and between-subjects factor of M + B dose (Vehicle, M + B). The analysis showed significant effects of DS (F1,33 = 43.0, p < 0.0001), M + B dose (F1,33 = 4.4, p = 0.0029), and DS x M + B dose (F1,33 = 4.3, p = 0.045), indicating that IL microinjections of M + B suppressed DS-controlled cocaine seeking (Fig. 2C, right panel). Bonferroni posthoc analysis showed that M + B decreased cocaine seeking during both DS+ trials (t33 = 2.8, p = 0.018) and DS− trials (t33 = 2.9, p = 0.013). Further, M + B did not affect discriminated cocaine seeking (Fig. S2) during the test (t33 = 0.03859, p = 0.96945).
Experiment 3: Microinjections of M + B into PL prior to DS-controlled cocaine seeking
Following discrimination training, we first tested rats for cocaine seeking on abstinence day 1 (Fig. 3C, left panel). We then placed the rats in their homecage for 20 days and tested whether M + B microinjections into PL would affect DS-controlled cocaine seeking on abstinence day 21. Mixed ANOVA analysis with within-subject factor of DS and between-subjects factor of M + B dose showed a significant main effect of DS (F1,28 = 100.4, p < 0.0001) but not M + B dose or interaction (p values>0.05), indicating that PL microinjections of M + B did not affect DS-controlled cocaine seeking (Fig. 3C, right panel).
Experiment 4: Microinjections of M + B into IL or PL prior to DS-controlled cocaine self-administration
Following discrimination training, we used a within-subjects design and tested for DS-controlled cocaine self-administration after counterbalanced vehicle and M + B microinjections into IL or PL (Fig. 4C). For each subregion, we analyzed the number of lever presses made during the test session using repeated measures ANOVA: within-subjects factors of DS and M + B dose. For both subregions, we observed a significant main effect of DS (PL: F1,10 = 82.8, p < 0.0001; IL: F1,11 = 57.6, p < 0.0001) but no effect of M + B dose or interaction (p values>0.05), indicating that inactivation of IL or PL did not affect DS-controlled cocaine self-administration.
Experiment 5: Effect of M + B on IL synaptic activity using ex vivo brain slice electrophysiology
We used whole cell voltage clamp recordings in an ex vivo brain slice preparation to determine the effect of GABAergic receptor agonism on IL synaptic activity. We used either paired pulses or a pulse train of electrical stimulation to elicit postsynaptic responses in visually identified Layer 5/6 pyramidal neurons (Fig. 5A). After establishing a stable baseline level of synaptic responding, we bath-applied M + B (0.03 nM muscimol + 0.3 nM baclofen) onto the slice. M + B application caused 76.1 ± 5.2 percent change in the amplitude of the synaptic response for EPSC1, 66.0 ± 7.2 percent change in EPSC2 and significantly increased paired-pulse response (t10 = 6.01, p = 0.0001). M + B application caused 68.5 ± 5.2 percent change in P1, 60.0 ± 6.6 percent change in P2, 55.4 ± 7.4 percent change in P3, 51.0 ± 8.0 percent change in P4, 48.3 ± 7.5 percent change in P5, 45.7 ± 8.7 percent change in P6, and 46.7 ± 8.7 percent change in P7. We also recorded spontaneous excitatory postsynaptic responses (sEPSCs) before and after drug application by recording continuously for 5 min during the baseline period and once again after drug application for 5–10 min (Fig. 5B). M + B application significantly reduced the frequency of sEPSCs in recorded neurons (t10 = 3.0, p = 0.013) and had no effect on sEPSC amplitude (t10 = 0.76, p = 0.466) (Fig. 5B, middle and right panel).
A Example traces showing evoked paired-pulse synaptic responses before and after drug application (upper left), and example traces showing synaptic responses to a train of seven pulses (upper right). Summary graph showing the percent amplitude change in the paired-pulse response after application of M + B (bottom left). Paired-pulse ratios before and after drug application (bottom middle). Summary graph showing the percent amplitude change in the pulse train response after M + B application (n = 11 cells, 9 rats). * indicates significant difference (p < 0.05) between vehicle and M + B groups. B Example traces showing spontaneous synaptic response before and after drug application (left). Summary graph showing the effect of M + B on sEPSC frequency (middle) and sEPSC amplitude (right) (n = 11 cells, 9 rats). * indicates significant difference (p < 0.05) between vehicle and M + B groups.
Discussion
We examined behavioral and neurobiological mechanisms underlying trial-based DS-controlled cocaine seeking and taking. We report four main findings. First, after 21 abstinence days, DS+ alone increased cocaine seeking relative to no DS trials while DS− decreased (DS+)-induced cocaine seeking when presented together with the DS+ in both-DSs trials. Second, IL (but not PL) M + B microinjections reduced cocaine seeking during both DS+ and DS− trials but did not affect discriminated responding. Third, IL or PL M + B microinjections did not affect ongoing DS-controlled cocaine self-administration. Finally, in mPFC slices M + B application suppressed the magnitude of electrically evoked postsynaptic responses and decreased spontaneous EPSC frequency. Overall, our data indicate that during abstinence, DS+ and DS− independently control the expression and suppression of DS-controlled drug seeking, and that DS-controlled cocaine seeking (but not discriminated responding) is mediated by IL activity, likely via a presynaptic mechanism.
Behavioral mechanisms underlying trial-based DS-controlled relapse to cocaine seeking
During discrimination training in our trial-based procedure [21], the two DSs are presented within the same session and set the occasion for responding (or not responding) on a common retractable lever. Thus, the only aspect that discriminates between trial types is the DS itself. In our previous study [21], we assessed cocaine seeking only during DS+ or DS− trials. Thus, it was unknown whether it is the presence of DS+ or absence of DS− that induces cocaine seeking. To address this question, we assessed the individual contributions of the DS+ and DS− to cocaine seeking, relative to presentation of the lever alone (no-DSs trials) or when both DSs were presented together (both-DSs trials). While the DS+ increased responding relative to no-DSs trials, the DS− did not alter responding relative to no-DSs trials, possibly due to a floor effect (responding was low in no-DSs trials). Critically, when both DSs were presented together (both-DSs trials), the DS− decreased cocaine seeking induced by the DS+ and served as a conditioned inhibitor of the DS+ [64]. We did not observe higher responding in no-DSs trials vs. DS− trials, indicating that increased responding in DS+ trials was not due to disinhibition of responding due to DS− removal. Overall, our data indicate that DS+ and DS− contribute independently to DS control of drug seeking during abstinence.
Role of mPFC activity in (DS+)-induced expression of cocaine seeking
IL (but not PL) microinjections of M + B decreased cocaine seeking during DS+ trials, indicating that (DS+)-induced cocaine seeking during abstinence is mediated by IL activity. Previous studies of (DS+)-induced cocaine seeking primarily used session-based procedures, where rats’ lever pressing was assessed in separate sessions while they were exposed continuously to either a DS+ or DS− [65, 66]. Weiss and colleagues found that Fos expression was higher in PL following DS+ (versus DS−) induced reinstatement after extinction, an effect reversed by systemic injections of SCH39166 (a dopamine D1/D5 receptor antagonist); IL activity was not assessed [65, 66]. However, the authors did not inactivate either region to assess their causal role in (DS+)-induced reinstatement.
Our results agree with those of Suto and colleagues who used the Daun02 inactivation procedure [67] and found a role for IL in (DS+)-induced reinstatement of food seeking following session-based self-administration and extinction training [68]. While these results suggest a general role for IL in (DS+)-induced relapse to reward seeking, additional studies using the same procedure and other reinforcers (e.g. food, heroin) are necessary to test this possibility.
Role of mPFC neural activity in (DS−)-based inhibitory control of cocaine seeking
Cocaine seeking during DS- trials did not increase following PL or IL M + B microinjections. However, these negative results should be interpreted with caution, because under our experimental conditions, the DS- did not inhibit cocaine seeking relative to the no-DSs trials. In a previous study where a signaled DS− decreased cocaine seeking, this effect was associated with increased Fos expression in PL [69]. Further, PL (but not IL) inactivation with muscimol reversed inhibitory DS− control [13]. In a different study, Daun02 inactivation of (DS−)-responsive IL Fos-labeled neurons (using a different procedure also containing only a signaled DS−), reversed the inhibitory effect of the DS− on cocaine and alcohol seeking [18]. In contrast, we observed decreased cocaine seeking during DS− trials following IL M + B microinjections. A possible explanation for this inhibitory effect is that IL M + B microinjections inhibited residual excitatory drive due to exposure to the common active lever during DS− presentation after 21 abstinence days. It is also possible that IL M + B microinjections decreased the time-dependent potentiation of cocaine seeking during abstinence (‘incubation’) [70, 71], because responding in the M + B group during both DS + and DS− trials on day 21 was similar to day 1 responding without intracranial injections; however, M + B microinjections prior to day 1 relapse test are necessary to verify this hypothesis. It is unlikely that this decrease is due to non-specific suppression of operant responding during the relapse test as IL M + B microinjections had no effect on (DS+)-controlled cocaine self-administration in our study. Additionally, previous studies have shown that IL M + B inactivation is ineffective at decreasing stress- or cocaine priming-induced reinstatement of cocaine seeking [72, 73], and after extinction, this manipulation potentiates spontaneous recovery [74] and reinstates cocaine seeking [40].
It is possible that previous studies detected mPFC contributions to inhibitory effects of DS− because training and testing were conducted under conditions where baseline responding (in the absence of DS−) was higher than in our task, likely due to the continued presence of excitatory stimuli such as cocaine availability prior to DS− presentation [13], response-contingent cocaine-paired CSs [18], or DS− presentation together with cocaine-predictive cues [13, 18]. This higher baseline would allow for an observable suppression of responding in the presence of the DS− that could then be manipulated pharmacologically. Thus, in these studies the no-DS− condition was likely more similar to our excitatory DS+ condition (vs. our no-DS condition) and it is possible that we would also have observed disinhibition of DS− control following our pharmacological manipulations if we had compared rats’ responding during trials with or without the DS−, in the presence of the excitatory DS+ in both conditions (i.e. both-DSs versus DS+ trials in Experiment 1).
Altogether, in studies where the DS− decreased cocaine seeking or taking, the IL and PL both appear to play a role in (DS−)-based inhibitory control. Future studies are necessary to examine IL and PL role in the inhibitory effect of DS− on cocaine seeking induced by DS+ in our trial-based procedure.
Role of mPFC neural activity in DS-controlled cocaine self-administration
IL or PL M + B microinjections had no effect on ongoing DS-controlled cocaine self-administration in our task. In contrast, Gutman et al. [20] found that IL and PL M + B microinjections prior to discriminated cocaine self-administration decreased responding during DS+ trials and increased responding during DS− trials. This study did not assess relapse to cocaine seeking during abstinence.
Both our task and the Gutman task employed discrete trials and counterbalanced presentations of DS+/−. However, in the Gutman study, lever-presses during DS+ trials (but not DS− trials) caused retraction of both levers and led to DS+ turning off. In contrast, in our task lever-presses were reinforced on an FR1 reinforcement schedule during DS+ (but not DS−) trials, the lever and DS+ stayed on for 60-s, and multiple infusions could be earned during each trial. While no explicit tone/light CS+ was paired with drug deliveries in the Gutman study, immediate lever retraction after a lever press only during DS+ trials likely served as a CS+ in their procedure. Additionally, the rats in the Gutman study were given initial food self-administration training, were limited to 2-h daily cocaine self-administration, and were allowed to respond only once during each 10-s DS+ trial to receive a single cocaine infusion. These differences in task structure and training methodology likely caused differential engagement of mPFC and also affected how DS+/− in the two studies exerted behavioral control during cocaine self-administration. Our results do agree, however, with those from Moorman and Aston-Jones who found a role for IL, but not PL, in DS+ control of discriminated sucrose self-administration [53].
Effects of GABA receptor agonists on mPFC neuronal activity
We performed whole-cell recordings of IL pyramidal neurons in layer 5/6 to determine synaptic mechanisms for the inhibitory effect of M + B on neuronal activity. We chose layer 5/6 instead of superficial layers 2/3 since this is the primary mPFC output layer and is most likely to affect downstream circuits and behavior [75]. We performed recordings with network activity intact (i.e., no synaptic blockers present) to keep recording conditions similar to the in vivo conditions and chose M + B concentrations similar to those used for mPFC microinjections. Although GABA receptor agonists are frequently used to inactivate brain regions of interest [28, 39, 40, 43, 48], their effect on mPFC pyramidal neuron synaptic activity has not been characterized. M + B application reduced amplitude of electrically evoked postsynaptic currents by ~75%. The apparent efficacy of M + B lessened over the course of a seven-pulse train at 25 Hz due primarily to a lessening of overall magnitude of postsynaptic responses without M + B and little change of postsynaptic responses with M + B. We observed increased paired-pulse ratio following M + B application. One putative mechanism is inhibition of presynaptic calcium release via activation of presynaptic GABAB receptors [76, 77]. The contribution of presynaptic GABAA receptors is more difficult to discern given that the ultimate effect of their activation depends on several factors, including modulation by surrounding GABAB receptors and overall GABAA receptor activation level [78]. M + B reduced frequency of spontaneous postsynaptic currents in most recorded neurons, but had no effect on amplitude of these currents, similar to a previous study [51].
Together, our data suggest that the predominating effect of M + B on synaptic transmission occurs via a presynaptic mechanism. Increased paired-pulse ratio coupled with decreased spontaneous event frequency support this conclusion. Since M + B effectively silenced synaptic activity onto IL neurons, it appears that excitatory synaptic activity onto IL neurons is necessary for DS-controlled cocaine seeking. Of note, our recordings were performed in brain slices of drug-naïve rats. Thus, while unlikely, we cannot rule out that modulation of synaptic activity by M + B is altered by cocaine experience [79, 80].
General role of PL versus IL neural activity in drug seeking
Early studies using the extinction-reinstatement model [81] where rats were trained to self-administer cocaine and exposed to discrete cues or cocaine priming to induce reinstatement of cocaine seeking led to the hypothesis that PL promotes drug seeking while IL suppresses drug seeking [34, 38,39,40, 82, 83]. At least for cocaine, a number of studies support this hypothesis, although recent evidence suggests that prior extinction training might be required to engage IL inhibitory control during cue- but not cocaine-primed reinstatement of cocaine seeking [58, 59, 84] (but see [60]). However, results from studies using the extinction-reinstatement model with heroin, alcohol, methamphetamine, and sucrose where reinstatement was induced by drug priming, discrete cues, and contextual cues did not support this hypothesis [42, 43, 51, 85,86,87]. Further, evidence that the PL-go/IL-stop hypothesis generalizes to other relapse-related models (that do not rely on extinction to suppress drug seeking) is mixed. Koya et al. [48]. showed that IL but not PL inactivation using M + B decreases cocaine seeking after 30 abstinence days. In contrast, Cameron et al. [60] showed that optogenetic activation of the IL to nucleus accumbens pathway suppresses cocaine seeking regardless of the period of abstinence. Additionally, PL neurons have been shown to encode incubated cocaine seeking [49] and projections from PL and IL to nucleus accumbens promote and inhibit incubation of cocaine seeking, respectively [61]. Finally, our studies using the Daun02 inactivation procedure suggest a role for IL ensembles in both promotion and inhibition of non-reinforced cocaine (and food) seeking [56, 57]. Together, these results do not support the hypothesis that PL and IL always play opposing roles in drug seeking.
Specifically regarding DS control of cocaine seeking, our finding that inhibition of IL but not PL activity decreases DS-controlled relapse after prolonged abstinence also does not support the PL-go/IL-stop hypothesis. Previous studies using different DS-based procedures also suggest a more complicated role, with evidence for IL involvement in promoting DS+ controlled food and cocaine seeking [20, 52, 53] and for both PL and IL in inhibition of reward seeking by DS− [13, 18, 20, 53].
One possibility for the discrepant results described above is that mPFC neural activity represents a higher order associative structure that underlies cue- and context-guided expression of learned behaviors rather than simply promoting or inhibiting these behaviors. Indeed, mPFC neurons respond not only during reward-related actions, but also to reward-associated contexts and cues, and to non-contingent reward delivery [37, 49, 53, 88,89,90,91]. In support of this hypothesis, and specifically for DS-control, Moorman and Aston-Jones [53] conducted electrophysiological recordings in PL and IL during a sucrose DS-task (before and during extinction) and found that neuronal activity in neither region is specifically locked to simply the DS or the action (i.e. going or stopping), but instead represents the appropriate behavioral action based on ‘context’ (e.g., responding during DS+ vs. inhibiting responses following extinction training). In most previous studies, only one ‘context’ (training or extinction; no DS or DS+) and associated action pair (press or withhold) was tested at a time, making it difficult to ascertain whether mPFC activity represented (1) the behavioral action measured, (2) the cue-response association needed to perform the correct action, or (3) some higher order information requiring input from other upstream brain regions.
In contrast, the rats in our study had to recognize and make appropriate responses during two orthogonal DS-action pairs. Under these conditions, we found that suppression of IL activity reduced DS-controlled cocaine seeking but did not lead to a breakdown of DS-guided discriminated responding. This suggests that the DS-cocaine association is likely processed in other regions upstream of the IL and that IL activity in our procedure integrates this information to support DS-controlled cocaine seeking during abstinence.
In line with the idea of mPFC processing higher order associative structures [91], it is likely that the complex computations needed for appropriate task performance in drug seeking are mediated by separate but intermingled neuronal ensembles within the mPFC that allow more flexible high-resolution responses to different conditions (e.g. cues, context, DS) than would be allowed by uniform action (promoting or suppressing) of all neurons in a brain area [92]. In support of this hypothesis, targeted ablation of only cue- and context-induced drug-seeking specific neurons is sufficient to disrupt ongoing promotion or suppression of reward seeking [18, 42, 56, 57, 68, 92,93,94,95,96,97,98,99,100,101,102]. Additionally, we and others have shown that separate ensembles within the same brain region can control opposing effects on drug and non-drug reward seeking [18, 56, 57, 68, 92, 102]. Based on our current findings and those of others, we hypothesize that future ensemble-level manipulations are likely to identify DS-drug-specific ensembles in regions upstream of mPFC, and cue-action-specific ensembles within the mPFC, that act together to induce the appropriate DS-controlled behavioral response.
Funding and disclosure
The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the text of the paper. The research was supported by funds from the Intramural Research Program of NIDA (grant no. DA000467-17). RM received funding from the NIH Center for Compulsive Behaviors. BJT received funding from NIDA (grant no. DA048530). ORD was supported by the NIDA IRP Scientific Director’s Fellowship for Diversity in Research.
References
O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J psychiatry. 2005;162:1423–31.
Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen psychiatry. 1973;28:611–6.
Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol psychiatry. 2011;69:708–11.
Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, et al. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addiction Biol. 2015;20:513–22.
Wang G, Shi J, Chen N, Xu L, Li J, Li P, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PloS ONE. 2013;8:e68791.
Parvaz MA, Moeller SJ, Goldstein RZ. Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry. 2016;73:1127–34.
Weiss F. Advances in animal models of relapse for addiction research. In: Kuhn CM, Koob GF, editors. Advances in the Neuroscience of Addiction. Boca Raton (FL). Taylor & Francis: CRC Press; 2010.
Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52.
Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23:675–83.
Ettenberg A. Haloperidol prevents the reinstatement of amphetamine-rewarded runway responding in rats. Pharmacol, Biochem, Behav. 1990;36:635–8.
Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–79.
McFarland K, Ettenberg A. Reinstatement of drug-seeking behavior produced by heroin-predictive environmental stimuli. Psychopharmacology (Berl). 1997;131:86–92.
Mihindou C, Guillem K, Navailles S, Vouillac C, Ahmed SH. Discriminative inhibitory control of cocaine seeking involves the prelimbic prefrontal cortex. Biol psychiatry. 2013;73:271–79.
Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–57.
Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23:7239–45.
Pitchers KK, Phillips KB, Jones JL, Robinson TE, Sarter M. Diverse roads to relapse: a discriminative cue signaling cocaine availability is more effective in renewing cocaine seeking in goal trackers than sign trackers and depends on basal forebrain cholinergic activity. J Neurosci. 2017;37:7198–208.
Ciccocioppo R, Martin-Fardon R, Weiss F. Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nat Neurosci. 2004;7:495–6.
Laque A, L De Ness G, Wagner GE, Nedelescu H, Carroll A, Watry D, et al. Anti-relapse neurons in the infralimbic cortex of rats drive relapse-suppression by drug omission cues. Nat Commun. 2019;10:3934.
Martin-Fardon R, Weiss F. Perseveration of craving: effects of stimuli conditioned to drugs of abuse versus conventional reinforcers differing in demand. Addiction Biol. 2017;22:923–32.
Gutman AL, Ewald VA, Cosme CV, Worth WR, LaLumiere RT. The infralimbic and prelimbic cortices contribute to the inhibitory control of cocaine-seeking behavior during a discriminative stimulus task in rats. Addiction Biol. 2017;22:1719–30.
Madangopal R, Tunstall BJ, Komer LE, Weber SJ, Hoots JK, Lennon VA, et al. Discriminative stimuli are sufficient for incubation of cocaine craving. eLife. 2019;8:e44427.
Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–36.
Kearns DN, Weiss SJ. Extinguished cocaine cues increase drug seeking when presented simultaneously with a non-extinguished cocaine cue. Drug Alcohol Depend. 2012;121:140–7.
Kearns DN, Tunstall BJ, Weiss SJ. Deepened extinction of cocaine cues. Drug Alcohol Depend. 2012;124:283–87.
Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Firing of nucleus accumbens neurons during the consummatory phase of a discriminative stimulus task depends on previous reward predictive cues. J Neurophysiol. 2004;91:1866–82.
Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol. 2004;91:1840–65.
Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008;155:573–84.
Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci. 2008;28:5088–98.
Kearns DN, Weiss SJ. Reinstatement of a food-maintained operant produced by compounding discriminative stimuli. Behav Process. 2005;70:194–202.
Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004;24:2923–33.
Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. II. Ensemble activity in orbitofrontal cortex. J Neurophysiol. 1995;74:751–62.
Lennon VA, Brenner MB, Weber SJ, Komer LE, Madangopal R. Trial-based discrimination procedure for studying drug relapse in rats. Bio-Protoc. 2019;9:e3445.
Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N. Y Acad Sci. 2007;1121:355–75.
Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35:276–84.
Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Front Neurosci. 2013;7:62.
Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–84.
Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–70.
Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–88.
LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–75.
Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–53.
Peters J, Vallone J, Laurendi K, Kalivas PW. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl). 2008;197:319–26.
Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, et al. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–2.
Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–91.
Calu DJ, Kawa AB, Marchant NJ, Navarre BM, Henderson MJ, Chen B, et al. Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. J Neurosci. 2013;33:214–26.
James MH, McGlinchey EM, Vattikonda A, Mahler SV, Aston-Jones G. Cued reinstatement of cocaine but not sucrose seeking is dependent on dopamine signaling in prelimbic cortex and is associated with recruitment of prelimbic neurons that project to contralateral nucleus accumbens core. Int J Neuropsychopharmacol. 2018;21:89–94.
Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl). 2013;229:453–76.
Khoo SY, Gibson GD, Prasad AA, McNally GP. How contexts promote and prevent relapse to drug seeking. Genes Brain Behav. 2017;16:185–204.
Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56:177–85.
West EA, Saddoris MP, Kerfoot EC, Carelli RM. Prelimbic and infralimbic cortical regions differentially encode cocaine-associated stimuli and cocaine-seeking before and following abstinence. Eur J Neurosci. 2014;39:1891–902.
Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56.
Caballero JP, Scarpa GB, Remage-Healey L, Moorman DE. Differential effects of dorsal and ventral medial prefrontal cortex inactivation during natural reward seeking, extinction, and cue-induced reinstatement. eNeuro. 2019;6:ENEURO.0296-19.2019.
Riaz S, Puveendrakumaran P, Khan D, Yoon S, Hamel L, Ito R. Prelimbic and infralimbic cortical inactivations attenuate contextually driven discriminative responding for reward. Sci Rep. 2019;9:3982.
Moorman DE, Aston-Jones G. Prefrontal neurons encode context-based response execution and inhibition in reward seeking and extinction. Proc Natl Acad Sci USA. 2015;112:9472–7.
Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:335–56.
Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res. 2015;1628:219–32.
Warren BL, Kane L, Venniro M, Selvam P, Quintana-Feliciano R, Mendoza MP, et al. Separate vmPFC ensembles control cocaine self-administration versus extinction in rats. J Neurosci. 2019;39:7394–407.
Warren BL, Mendoza MP, Cruz FC, Leao RM, Caprioli D, Rubio FJ, et al. Distinct Fos-expressing neuronal ensembles in the ventromedial prefrontal cortex mediate food reward and extinction memories. J Neurosci. 2016;36:6691–703.
Müller Ewald VA, De Corte BJ, Gupta SC, Lillis KV, Narayanan NS, Wemmie JA, et al. Attenuation of cocaine seeking in rats via enhancement of infralimbic cortical activity using stable step-function opsins. Psychopharmacology (Berl). 2019;236:479–90.
Augur IF, Wyckoff AR, Aston-Jones G, Kalivas PW, Peters J. Chemogenetic activation of an extinction neural circuit reduces cue-induced reinstatement of cocaine seeking. J Neurosci. 2016;36:10174–80.
Cameron CM, Murugan M, Choi JY, Engel EA, Witten IB. Increased cocaine motivation is associated with degraded spatial and temporal representations in IL-NAc neurons. Neuron. 2019;103:80–91.e7.
Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–67.
Marchant NJ, Campbell EJ, Pelloux Y, Bossert JM, Shaham Y. Context-induced relapse after extinction versus punishment: similarities and differences. Psychopharmacology (Berl). 2019;236:439–48.
National Research Council Committee for the Update of the Guide for the C, Use of Laboratory A. The National Academies Collection: Reports funded by National Institutes of Health. Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US) Copyright © 2011, National Academy of Sciences.; 2011.
Kearns DN, Weiss SJ, Schindler CW, Panlilio LV. Conditioned inhibition of cocaine seeking in rats. J Exp Psychol Anim Behav Process. 2005;31:247–53.
Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–81.
Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–72.
Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–73.
Suto N, Laque A, De Ness GL, Wagner GE, Watry D, Kerr T, et al. Distinct memory engrams in the infralimbic cortex of rats control opposing environmental actions on a learned behavior. Elife. 2016;5:5.
Navailles S, Guillem K, Vouillac-Mendoza C, Ahmed SH. Coordinated recruitment of cortical–subcortical circuits and ascending dopamine and serotonin neurons during inhibitory control of cocaine seeking in rats. Cereb Cortex. 2014;25:3167–81.
Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47:214–26.
Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2.
McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63.
McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–60.
Nakamura Y, Tanaka T, Wakimoto Y, Noda K, Kuwahara Y. Alkaline phosphatase activity in the osteoclasts induced by experimental tooth movement. J Electron Microsc (Tokyo). 1991;40:403–6.
Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–31.
Takahashi T, Kajikawa Y, Tsujimoto T. G-Protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor. J Neurosci. 1998;18:3138–46.
Wu LG, Saggau P. GABAB receptor-mediated presynaptic inhibition in guinea-pig hippocampus is caused by reduction of presynaptic Ca2+ influx. J Physiol. 1995;485:649–57.
Khatri SN, Wu WC, Yang Y, Pugh JR. Direction of action of presynaptic GABA(A) receptors is highly dependent on the level of receptor activation. J Neurophysiol. 2019;121:1896–905.
Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17:351–65.
Dong Y, Taylor JR, Wolf ME, Shaham Y. Circuit and Synaptic Plasticity Mechanisms of Drug Relapse. J Neurosci. 2017;37:10867–76.
Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl). 2003;168:3–20.
Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–9.
Gourley SL, Taylor JR. Going and stopping: Dichotomies in behavioral control by the prefrontal cortex. Nat Neurosci. 2016;19:656–64.
Muller Ewald VA, LaLumiere RT. Neural systems mediating the inhibition of cocaine-seeking behaviors. Pharmacol, Biochem, Behav. 2018;174:53–63.
Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–88.
Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37:259–68.
Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–9.
Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proc Natl Acad Sci USA. 2012;109:5086–91.
Burgos-Robles A, Bravo-Rivera H, Quirk GJ. Prelimbic and infralimbic neurons signal distinct aspects of appetitive instrumental behavior. PloS ONE. 2013;8:e57575.
Peters YM, O’Donnell P, Carelli RM. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse. 2005;56:74–83.
Moorman DE, James MH, McGlinchey EM, Aston-Jones G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2015;1628:130–46.
Warren BL, Suto N, Hope BT. Mechanistic resolution required to mediate operant learned behaviors: insights from neuronal ensemble-specific inactivation. Front Neural Circuits. 2017;11:28.
Brebner LS, Ziminski JJ, Margetts-Smith G, Sieburg MC, Reeve HM, Nowotny T, et al. The emergence of a stable neuronal ensemble from a wider pool of activated neurons in the dorsal medial prefrontal cortex during appetitive learning in mice. J Neurosci. 2020;40:395–410.
Whitaker LR, Carneiro de Oliveira PE, McPherson KB, Fallon RV, Planeta CS, Bonci A, et al. Associative learning drives the formation of silent synapses in neuronal ensembles of the nucleus accumbens. Biol psychiatry. 2016;80:246–56.
Whitaker LR, Warren BL, Venniro M, Harte TC, McPherson KB, Beidel J, et al. Bidirectional modulation of intrinsic excitability in rat prelimbic cortex neuronal ensembles and non-ensembles after operant learning. J Neurosci. 2017;37:8845–56.
Cruz FC, Babin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y, et al. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci. 2014;34:7437–46.
Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schönig K, et al. Losing control: excessive alcohol seeking after selective inactivation of cue-responsive neurons in the infralimbic cortex. J Neurosci. 2015;35:10750–61.
de Guglielmo G, Crawford E, Kim S, Vendruscolo LF, Hope BT, Brennan M, et al. Recruitment of a neuronal ensemble in the central nucleus of the amygdala is required for alcohol dependence. J Neurosci. 2016;36:9446–53.
Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, et al. Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci. 2017;37:1014–27.
Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Lê AD. Role of central amygdala neuronal ensembles in incubation of nicotine craving. J Neurosci. 2016;36:8612–23.
Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci. 2012;32:11600–9.
Kane L, Venniro M, Quintana‐Feliciano R, Madangopal R, Rubio FJ, Bossert JM. et al. Fos-expressing neuronal ensemble in rat ventromedial prefrontal cortex encodes cocaine seeking but not food seeking in rats. Addiction Biol. 2021;26:e12943
Acknowledgements
The authors thank Dr. David H. Epstein for statistical input and for thoughtful comments during the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
R.M., L.A.R., B.J.T., J.M.B., Y.S., and B.T.H. designed the experiments; R.M., L.A.R., B.J.T., J.M.B., S.J.W., M.B.B., V.A.L., O.R.D., and L.E.K. ran the experiments and collected the data; R.M., L.A.R., B.J.T., S.J.W., M.B.B., V.A.L., O.R.D., and L.E.K. analyzed the data; R.M., L.A.R., B.J.T., Y.S., and B.T.H. wrote the paper. All authors reviewed and approved the final version prior to submission.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Madangopal, R., Ramsey, L.A., Weber, S.J. et al. Inactivation of the infralimbic cortex decreases discriminative stimulus-controlled relapse to cocaine seeking in rats. Neuropsychopharmacol. 46, 1969–1980 (2021). https://doi.org/10.1038/s41386-021-01067-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01067-6
This article is cited by
-
Single-nucleus genomics in outbred rats with divergent cocaine addiction-like behaviors reveals changes in amygdala GABAergic inhibition
Nature Neuroscience (2023)
-
Prelimbic and infralimbic medial prefrontal cortex neuron activity signals cocaine seeking variables across multiple timescales
Psychopharmacology (2023)
-
Possible Involvement of Perineuronal Nets in Anti-Depressant Effects of Electroacupuncture in Chronic-Stress-Induced Depression in Rats
Neurochemical Research (2023)