Abstract
There is increasing evidence that skeletal muscle microvascular (capillary) blood flow plays an important role in glucose metabolism by increasing the delivery of glucose and insulin to the myocytes. This process is impaired in insulin-resistant individuals. Studies suggest that in diet-induced insulin-resistant rodents, insulin-mediated skeletal muscle microvascular blood flow is impaired post-short-term high fat feeding, and this occurs before the development of myocyte or whole-body insulin resistance. These data suggest that impaired skeletal muscle microvascular blood flow is an early vascular step before the onset of insulin resistance. However, evidence of this is still lacking in humans. In this review, we summarise what is known about short-term high-calorie and/or high-fat feeding in humans. We also explore selected animal studies to identify potential mechanisms. We discuss future directions aimed at better understanding the ‘early’ vascular mechanisms that lead to insulin resistance as this will provide the opportunity for much earlier screening and timing of intervention to assist in preventing type 2 diabetes.
Similar content being viewed by others
Introduction
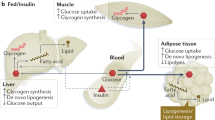
Glucose homoeostasis is maintained by the actions of various tissues such as the brain, skeletal muscle, kidneys, blood cells, splanchnic organs and adipose tissue [1, 2]. However, the vasculature is another tissue required for optimal glucose and hormone delivery to target tissues [3]. Blood flow through the capillaries in skeletal muscle, also known as microvascular blood flow (MBF), is particularly important because it is responsible for the exchange of glucose, insulin and other nutrients from the blood and/or plasma to the myocyte [4]. In healthy individuals, skeletal muscle MBF increases in response to insulin or following a meal which facilitates glucose disposal [5]. In contrast, this vascular process is impaired during states of insulin resistance which is evident in obesity and type 2 diabetes (T2D) and leads to reduced muscle glucose uptake [6,7,8]. Therefore, impaired muscle MBF is an important contributor to skeletal muscle insulin resistance and increases the risk of pre-diabetes and T2D.
In pathological conditions such as overweight, obesity and T2D, individuals have impaired insulin and post-meal MBF responsiveness [9,10,11]. These groups often have elevated circulating free fatty acids (FFAs) [7] and ectopic fat deposition in various tissues [12, 13] and throughout the vasculature [11] which may contribute to impaired MBF and insulin resistance [14]. Healthy animals fed a high-fat diet (HFD), even with moderately raised fat content (1.8-fold) or for a short duration (3 days), have reduced MBF responses to the insulin that occurs before the development of obesity or myocyte/whole-body insulin resistance [7, 15]. However, what is not clear is if diet-induced insulin resistance impairs skeletal muscle MBF in humans and if this occurs before the signs of increased adiposity or insulin resistance. A better understanding of the early vascular defects that lead to insulin resistance and glucose intolerance will provide the opportunity for much earlier screening and interventions to prevent T2D.
Diets such as high calorie (HC) and/or HFD present a valid model to study diet-induced insulin resistance [16,17,18,19]. In this review, we summarise what is known about the effects of short-term HC and/or HFD on insulin action, glucose metabolism and on MBF in humans. We also explore selected animal studies to highlight possible mechanisms. This review aims to identify important gaps in the current literature and provide direction for future research in order to develop successful dietary strategies to prevent insulin resistance and pre-diabetes resulting from HC and/or high-fat feeding.
Role of skeletal muscle blood flow in glucose metabolism
The vascular system can be categorised into two subgroups, the macrovasculature and microvasculature. The macrovasculature is inclusive of larger branches of vessels consisting of the arteries and veins, whereas the microvasculature includes the smallest branches of vessels that form the capillary networks that are embedded within a tissue [4]. The capillaries are thin-walled (often single cell) which allows for the exchange of nutrients, hormones and gases. Insulin is a key player in skeletal muscle macrovascular blood flow, MBF and myocyte glucose uptake [20, 21]. However, changes in MBF are not always mirrored by comparable changes in macrovascular flow as some studies have reported increases in total blood flow without any changes in MBF and vice versa [21, 22]. These findings have been made possible by the development of a number of techniques to measure macro- and micro-vascular blood flow (detailed in Table 1 and extensively reviewed elsewhere [23]. Changes in macrovascular blood flow occur after insulin’s action on increasing glucose disposal in skeletal muscle, suggesting a temporal dissociation between total limb blood flow and muscle glucose metabolism [24]. Insulin-dependent increases in MBF precede that of total limb blood flow and can occur at lower insulin concentrations [25, 26]. Our research team have also shown that insulin-stimulated increases in MBF can occur independent of changes in macrovascular blood flow but changes in MBF are rapid, and therefore MBF may facilitate the early increase in glucose uptake [4, 26].
Insulin-dependent increases in skeletal muscle MBF are regulated by the balance of nitric oxide (a potent vasodilator) and endothelin-1 (a potent vasoconstrictor) which are produced via complex biochemical pathways [4]. Nitric oxide is produced by the endothelial cells in response to insulin, and diffuses into the adjacent smooth muscle cells, causing relaxation and resulting in vessel dilation and increased capillary surface area of downstream capillaries [4]. As a result, glucose disposal to the tissues is enhanced due to increased delivery of glucose and insulin to the myocyte. Insulin-stimulated microvascular perfusion is seen whether insulin is secreted from the pancreas (e.g. during a mixed meal challenge) or via exogenous insulin infusion (e.g. during hyperinsulinemic–euglycemic clamp) [4]. Blocking insulin-mediated increases in muscle MBF leads to a reduction of muscle-specific glucose disposal by ~40% when assessed using the hyperinsulinemic–euglycemic clamp technique [27], which provides evidence of the role of MBF in glucose disposal. Insulin’s microvascular actions are acutely blunted with vasoconstrictive agents (e.g. α-methylserotonin [28] and endothelin-1 [29]) and inflammatory cytokines (e.g. tumour necrosis alpha [30]) which consequently causes insulin resistance. Importantly, the infusion of vasodilators during hyperinsulinemic conditions does not always overcome microvascular insulin resistance or improve insulin sensitivity and glucose disposal in muscle despite increased macrovascular blood flow [31]. This indicates that macrovascular blood flow is sometimes permissive rather than stimulatory for glucose disposal. For example, vasodilation with methacholine in the presence of insulin in rats stimulates MBF and skeletal muscle glucose uptake, whereas, a similar degree of vasodilation with bradykinin does not [32]. Treatments that augment insulin-stimulated MBF also improve insulin sensitivity and these include: metformin [33], 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (activator of AMP-activated protein kinase) [34], glucagon-like peptide-1 [35], sodium salicylate [36] and exercise training [37, 38]. Therefore, it is well established that impaired skeletal muscle MBF is a contributor to insulin resistance, and improvements in MBF can augment insulin sensitivity. Understanding what factors (including diet) contribute to microvascular insulin resistance is important for preventing the development of pre-diabetes and T2D.
Effect of short-term high calorie and/or high-fat diet in inducing insulin resistance
The literature consistently reports that short-term HC, HC with high-fat (HCHF) or HFD impair insulin action as measured by glucose tolerance tests, mixed meal challenges or hyperinsulinemic–euglycemic clamp (Table 2), confirming that high-fat and/or HC diets are a suitable model to study diet-induced insulin resistance. For example, Durrer et al. [39] showed a 17% increase in glucose area under the curve during an oral glucose tolerance test (OGTT) after 7 days on an HFD. Morrison et al. [40] reported a 14% increase in post-prandial glucose area under the curve and a 31% increase in post-prandial insulin area under the curve following a mixed meal challenge after 28 days on an HC diet. Similarly, a 7-day HCHF feeding in both men and women also increases the post-prandial glucose area under the curve and insulin area under the curve in response to a mixed meal challenge [19, 41]. The increased area under the curve for glucose and insulin suggests glucose intolerance alongside insulin resistance as a result of overfeeding. Insulin resistance has also been demonstrated by an increased homoeostatic assessment model of insulin resistance (HOMA-IR) in as early as 5 days [42] and 7 days [41, 43,44,45]. Reduced insulin sensitivity as a result of HC and HCHF feeding has also been identified using the hyperinsulinemic–euglycemic clamp technique [17, 45,46,47].
Some studies report no changes in insulin action with overfeeding [48,49,50,51,52]. These inconsistent findings could be due to a large variation in the overfeeding protocols used in different studies. For example, studies with HC interventions have increased energy intakes ranging from +40% to +150%, and studies with HF interventions have total fat intakes ranging from 37% and 82% of total energy (Table 2). This wide range of overfeeding protocols may lead to differing metabolic loads and contribute to inconsistent glucose and insulin outcomes. There are also other limitations in regard to study design including small sample sizes in some studies (for example some studies only investigated 6 participants) [45, 49], large variations in the age range of participants (Bakker et al. [47] investigated 19 to 26-year-old participants, whereas Boden et al. [45] investigated 46 to 55-year-old participants), uneven distribution of males to females (with many studies predominantly recruiting males [40, 42,43,44,45,46,47, 51]), all of which are factors that may have contributed to variations in study outcomes. Nonetheless, most studies do suggest that short-term HC/HCHF/HF diets induce glucose intolerance and insulin resistance in humans.
Mechanisms for impaired glucose metabolism and insulin action
Both human and animal studies suggest various mechanisms by which short-term overfeeding can impair glucose tolerance and insulin sensitivity. One of these mechanisms involves increased circulating FFAs, greater adiposity and ectopic fat deposits in skeletal muscle and the vasculature per se. Boden et al. [45] reported an increase in both body weight and total body fat by 3.5 kg after 7 days of HC feeding (+150% calories with 35% of total energy from fat). Increases in calories or fat consumption are linked to hyperplasia and hypertrophy of the adipocytes thereby causing increased fat mass and weight gain [53]. Hypertrophic adipocytes exhibit reduced blood flow leading to a greater hypoxic and inflammatory cellular environment [54]. The inflammation also results from increased macrophage infiltration of the adipose tissue and dysfunctional cytokine/adipokine production [55] which include leptin, tumour necrosis alpha, interleukin-6, interleukin-8, interleukin-1 and monocyte chemoattractant protein-1. The increased inflammatory environment may cause insulin resistance and reduced glucose uptake in insulin-sensitive tissues (muscle and liver), thereby causing glucose intolerance.
Lipid accumulation in the skeletal muscle as a result of overfeeding may be another mechanism for impaired glucose metabolism and insulin action. Andrich et al. [56] reported that after 14 days of HFD (61% of total energy) the percent area and the average size of intramyocellular lipid droplets were significantly increased in the soleus muscle of HFD-fed rats. In line with these results, Wardle et al. [52] reported that a HCHF diet (150% energy with 60% total energy from fat) for 6 days significantly increased the accumulation of ceramides by 1.4 fold in the skeletal muscle in humans [52]. Skeletal muscle ceramide content is closely linked with insulin resistance in skeletal muscle [57]. Boon et al. [42] reported that insulin resistance induced by 5 days of HCHF feeding (as assessed by HOMA-IR) also increased the expression of various macrophage markers (for example cluster of differentiation CD68, CD14 and CD11c) in skeletal muscle and reduced the markers of insulin signalling (solute carrier family transporter SLC2A and glycogen synthase-1). Therefore a disruption in the insulin signalling pathway could be another mechanism for diet-induced insulin resistance. A limitation of this study is the investigation of mRNA expression of insulin signalling genes which are not always reflective of protein function and enzyme activity.
Oxidative stress induced by overfeeding can also disrupt insulin signalling [45]. Oxidative stress is associated with several glucose transporter-4 posttranslational modifications in particularly carbonylation (alteration of protein function) which may lead to impaired insulin-stimulated glucose transport [45]. Boden et al. [45] showed that oxidative stress inhibits insulin signalling by inactivating insulin receptor substrate 1/2 and Adocchio et al. [48] identified increases in skeletal muscle serine phosphorylation of insulin receptor substrate-1 in healthy participants who were given an HCHF diet (40% increase in energy with 50% of total energy from fat) for 5 days. Degradation (by 35%) of the intracellular insulin receptor was demonstrated after 10 days of HFD (67% of total energy) in rodents alongside reduced muscle glucose uptake [58].
Overall, despite short-term overfeeding inducing minor weight gain, it does not consistently impair fasting glucose and/or fasting insulin levels. However, overfeeding does impair functional outcomes of glucose tolerance and insulin action in humans as measured postprandially or during hyperinsulinemic–euglycemic clamp. However, it is not known if diet-induced insulin resistance impairs skeletal muscle blood flow in humans.
Effect of short-term high calorie and/or high-fat diet on skeletal muscle blood flow
Macrovascular blood flow
The literature investigating the effects of a short-term HC, HFD and/or HCHF feeding on the large artery or macrovascular blood flow in humans is sparse. Bui et al. [59] reported that the ingestion of a single high-fat meal (with 50 g of fat), compared to a low-fat meal (with 5.1 g of fat), significantly reduced total forearm blood flow (19.3%) as measured by venous occlusion plethysmography in healthy participants. Flow-mediated dilation (FMD) is an indicator of vascular endothelial health and has been investigated in acute high fat meal ingestion studies (single meal) [60, 61] and short-term HFD studies [39, 62]. Single high-fat meal studies report no change in FMD with the amount of fat ranging from 50–90g [60, 61]. Durrer et al. [39] showed reduced FMD and impaired glucose tolerance after 7 days of HFD (71% of total energy) in healthy participants. Keogh et al. [62] showed that HFD (with high saturated fat) reduces FMD by 50% within 3 weeks. Overall, despite limited research on the effect of short-term overfeeding on macrovascular blood flow in humans, studies looking at large artery dilation suggest that a short-term HFD may impair endothelial function. The reduced endothelial function has been linked to reduced insulin-mediated nitric oxide production and reduced muscle glucose uptake [3].
Microvascular blood flow (MBF)
It is known that individuals who have raised plasma FFA levels become insulin-resistant and have impaired skeletal muscle MBF responses to insulin [6, 63]. Several studies have investigated the effects of lipid infusion on MBF in humans and report impaired insulin-mediated MBF responses [63,64,65,66]. For example, one of the studies used lipid infusion (Intralipid plus heparin) to raise plasma FFAs, and investigated subsequent effects on insulin (n = 23) and meal (n = 10) related MBF and compared it to saline infusion as a control [6]. The authors showed that 3 h after saline infusion, both mixed meal challenge and insulin infusion (hyperinsulinemic–euglycemic clamp) increased insulin levels and stimulated MBF, as measured using the contrast-enhanced ultrasound (CEU) method (Table 1 for method details). However, 3 h after lipid infusion (which raised plasma FFA by 18-fold) MBF was blocked during both the mixed meal challenge and the insulin clamp despite an increase in insulin concentrations. The authors also observed decreased forearm insulin-stimulated glucose disposal (during the clamp) and elevated plasma glucose during the mixed meal challenge demonstrating insulin resistance [6]. This study provides evidence for a link between circulating lipids and impaired skeletal muscle microvascular function. However, lipid infusion is not a physiological model, and as highlighted in Table 2, there is a significant gap in the literature regarding the effects of diet-induced insulin resistance on skeletal muscle MBF in humans.
To our knowledge, there is only one human study that looked at the effect of HC feeding on skeletal muscle MBF [51]. This study fed healthy men a HC diet (60% increase in calories with 25% from fat) for an average of 29 days. The HC diet increased body weight by 3.5 kg but there was no change in fasting glucose, insulin or insulin sensitivity as assessed via hyperinsulinemic–euglycemic clamp [51]. However, the authors reported an impairment in the normal insulin-mediated increase in muscle MBF measured by CEU. Interestingly, the MBF in adipose tissue increased suggesting the body is directing the excess nutrients to adipose for storage and protecting muscle from insulin resistance [51]. The fact that the insulin sensitivity was not altered suggests that vascular insulin resistance may be an early event that happens before whole-body insulin resistance. A time-course investigation in this model would be beneficial to confirm the timing of vascular versus muscle and whole-body insulin resistance.
In rodents, Premilovac et al. [7] and St-Pierre et al. [8] found that 4 weeks of HFD led to impairments in skeletal muscle MBF (assessed using the 1-methylxanthine method) and insulin sensitivity measured via hyperinsulinemic–euglycemic clamp. Kubota et al. [15] demonstrated that HFD-fed mice have impaired microvascular perfusion (assessed using CEU) in response to insulin and this coincided with whole-body and muscle-specific insulin resistance. The temporal association between MBF and glucose metabolism in rodents was demonstrated by Zhao et al. [67]. They found that an HFD (60% total energy from fat) in rodents reduced insulin-stimulated microvascular perfusion (assessed using the CEU method) in as early as 3 days which became progressively worse after 1, 2 and 4 weeks [67]. The corresponding impairments in whole-body glucose disposal were observed only after 1 week (and not 3 days) which suggests impairments in MBF occur before impairments in glucose disposal. As such, short-term high-fat feeding in rodents leads to impairment in MBF responses to insulin, and microvascular insulin resistance occurs before metabolic insulin resistance.
Mechanisms for impaired blood flow
It is suggested that elevated FFAs and the accumulation of lipid in tissues like muscle and liver leads to disruption in insulin signalling, causing insulin resistance and impaired endothelial function [68]. Protein kinase B (Akt) signalling in endothelial cells plays a crucial role in the regulation of vascular homoeostasis. It also stimulates the expression and activity of endothelial nitric oxide synthase (eNOS) and improves endothelial function [69]. Parry et al. [41] reported that 7 days of HC feeding (45% increase in total energy) reduced insulin-stimulated eNOS Ser-phosphorylation in terminal arterioles of skeletal muscle and reduced glucose clearance in healthy participants. Parry et al. [41] suggested that reduced eNOS may have reduced nitric oxide production thereby reducing skeletal muscle MBF causing impaired glucose metabolism, although they did not specifically measure blood flow. Zhao et al. using a rodent model showed that one week of HFD leads to insulin resistance during a hyperinsulinemic-euglycemic clamp, impaired muscle MBF (assessed via CEU), abolished insulin-stimulated Akt and eNOS phosphorylation and increased inflammation in the aorta but not in muscle [67]. When the authors pharmacologically reduced inflammation, the microvascular function was restored, suggesting that inflammation plays a role in the development of microvascular dysfunction in HFD-fed animals. Chai et al. [70] showed that 4 weeks of HFD (60% of total energy from fat) significantly blunted the insulin-mediated increase in plasma nitric oxide and increased the levels of plasma endothelin-1 which is a potent vasoconstrictor. Other animal studies show that hormones like glucagon-like peptide-1 (GLP-1) and the globular form of adiponectin can restore impaired muscle MBF impairments and improve glucose uptake via nitric oxide-dependent mechanisms [71, 72].
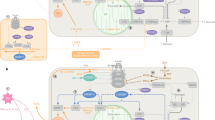
Therefore, findings within animal models suggest that HFD leads to increases adipose tissue mass, and inflammation, reduces eNOS and nitric oxide production and increases endothelin-1 levels. All these factors impair insulin-mediated muscle MBF and thereby impair glucose homoeostasis (Fig. 1). Interestingly, the HFD-induced impairments in microvascular perfusion were of similar magnitude to the impairments in the endothelium specific insulin receptor substrate-2 knock-out model which also has impaired insulin signalling in the endothelium and reduced eNOS activation [15].
TE, total energy, HCHF, high-calorie high fat, FFA, free fatty acids, TNF-α, tumour necrosis alpha, NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells, GLP-1, glucagon-like peptide, gAd, globular adiponectin, Akt, protein kinase B, eNOS, endothelial nitric oxide synthase, ET-1, endothelin-1, MBF, microvascular blood flow, ROS, reactive oxygen species, T2D, type 2 diabetes.
However, whether HC and/or HF feeding impairs insulin action in humans via impaired skeletal muscle MBF is still not clear. If these diets do impair MBF in healthy humans this would suggest there is a population group that is apparently “healthy” but at a high risk of developing insulin resistance and T2D. This group would be an ideal candidate for future dietary intervention and provide a driver for further investigation to prevent vascular insulin resistance caused by short-term HC and/or HF feeding.
Conclusions and future directions
Current evidence supports a potential link between microvascular dysfunction and HC, HCHF or HFD-induced insulin resistance. However, the majority of evidence is derived from rodent research with very few studies conducted in humans. This is important to research further in humans, as vascular insulin resistance caused by overfeeding may occur before whole-body insulin resistance and the subsequent development of chronic diseases including T2D. A better understanding of the very early vascular defects that lead to insulin resistance and glucose intolerance in humans would provide the opportunity for earlier screening and appropriate interventions to prevent diet-induced T2D.
Current research on diet-induced insulin resistance in humans has some limitations such as the use of a wide range of dietary intervention protocols (% total energy and % of fat contribution to total energy), use of non-physiological methods of assessing glucose tolerance (eg. OGTT), use of methods that only measure large artery function and not muscle-specific MBF, and studies with an uneven male to female participant ratio. Nonetheless, one study in humans showed HC diet for 29 days impaired the normal insulin-mediated increase in muscle MBF but not insulin sensitivity. Future studies should use modern techniques in vascular imaging (e.g., CEU) with robust and physiologically relevant study design (e.g., mixed meal challenge rather than OGTT) with both male and female participants to advance this field of research. Analysis of blood/plasma and tissue biopsies from human participants with diet-induced insulin resistance is required to understand whether inflammation, oxidative stress and reduced nitric oxide synthesis are contributory mechanisms in humans. Confirming if vascular insulin resistance is an early step in whole-body insulin resistance in humans will be a major step forward in the field of targeting and designing strategies to prevent insulin resistance and T2D.
References
Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–86.
Gerich JE. Physiology of glucose homeostasis. Diabetes Obes Metab. 2000;2:345–50.
Keske MA, Premilovac D, Bradley EA, Dwyer RM, Richards SM, Rattigan S. Muscle microvascular blood flow responses in insulin resistance and ageing. J Physiol. 2016;594:2223–31.
Keske MA, Dwyer RM, Russell RD, Blackwood SJ, Brown AA, Hu D, et al. Regulation of microvascular flow and metabolism: an overview. Clin Exp Pharm Physiol. 2017;44:143–9.
Meijer RI, De Boer MP, Groen MR, Eringa EC, Rattigan S, Barrett EJ, et al. Insulin-induced microvascular recruitment in skin and muscle are related and both are associated with whole-body glucose uptake. Microcirculation. 2012;19:494–500.
Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab. 2009;94:3543–9.
Premilovac D, Bradley EA, Ng HLH, Richards SM, Rattigan S, Keske MA. Muscle insulin resistance resulting from impaired microvascular insulin sensitivity in Sprague Dawley rats. Cardiovasc Res. 2013;98:28–36.
St-Pierre P, Genders AJ, Keske MA, Richards SM, Rattigan S. Loss of insulin-mediated microvascular perfusion in skeletal muscle is associated with the development of insulin resistance. Diabetes Obes Metab. 2010;12:798–805.
Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–42.
Emanuel AL, de Clercq NC, Koopen AM, van Poelgeest E, Serlie MJM, van Raalte DH, et al. Iloprost infusion prevents the insulin-induced reduction in skeletal muscle microvascular blood volume but does not enhance peripheral glucose uptake in type 2 diabetic patients. Diabetes Obes Metab. 2018;20:2523–31.
Meijer RI, Serne EH, Korkmaz HI, van der Peet DL, de Boer MP, Niessen HW, et al. Insulin-induced changes in skeletal muscle microvascular perfusion are dependent upon perivascular adipose tissue in women. Diabetologia 2015;58:1907–15.
Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes (Lond). 2019;43:139–48.
Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–8.
Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ. Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care. 2009;32:1672–7.
Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307.
Cornier M-A, Bergman BC, Bessesen DH. The effects of short-term overfeeding on insulin action in lean and reduced-obese individuals. Metabolism. 2006;55:1207–14.
Tam CS, Viardot A, Clément K, Tordjman J, Tonks K, Greenfield JR, et al. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes. 2010;59:2164.
Samocha-Bonet D, Campbell LV, Mori TA, Croft KD, Greenfield JR, Turner N, et al. Overfeeding reduces insulin sensitivity and increases oxidative stress, without altering markers of mitochondrial content and function in humans. PLoS One. 2012;7:e36320.
Parry SA, Smith JR, Corbett TR, Woods RM, Hulston CJ. Short-term, high-fat overfeeding impairs glycaemic control but does not alter gut hormone responses to a mixed meal tolerance test in healthy, normal-weight individuals. Br J Nutr. 2017;117:48–55.
Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990;85:1844–52.
Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes. 1997;46:1381.
Clark AD, Barrett EJ, Rattigan S, Wallis MG, Clark MG. Insulin stimulates laser Doppler signal by rat muscle in vivo, consistent with nutritive flow recruitment. Clin Sci (Lond). 2001;100:283–90.
Gliemann L, Mortensen SP, Hellsten Y. Methods for the determination of skeletal muscle blood flow: development, strengths and limitations. Eur J Appl Physiol. 2018;118:1081–94.
Fugmann A, Lind L, Andersson PE, Millgard J, Hanni A, Berne C, et al. The effect of euglucaemic hyperinsulinaemia on forearm blood flow and glucose uptake in the human forearm. Acta Diabetol. 1998;35:203–6.
Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–23.
Vincent MA, Dawson D, Clark AD, Lindner JR, Rattigan S, Clark MG, et al. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes. 2002;51:42–8.
Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–9.
Rattigan S, Clark MG, Barrett EJ. Acute vasoconstriction-induced insulin resistance in rat muscle in vivo. Diabetes. 1999;48:564–9.
Ross RM, Kolka CM, Rattigan S, Clark MG. Acute blockade by endothelin-1 of haemodynamic insulin action in rats. Diabetologia 2007;50:443–51.
Youd JM, Rattigan S, Clark MG. Acute impairment of insulin-mediated capillary recruitment and glucose uptake in rat skeletal muscle in vivo by TNF-alpha. Diabetes 2000;49:1904–9.
Nuutila P, Raitakari M, Laine H, Kirvela O, Takala T, Utriainen T, et al. Role of blood flow in regulating insulin-stimulated glucose uptake in humans. Studies using bradykinin, [15O]water, and [18F]fluoro-deoxy-glucose and positron emission tomography. J Clin Invest. 1996;97:1741–7.
Mahajan H, Richards SM, Rattigan S, Clark MG. Local methacholine but not bradykinin potentiates insulin-mediated glucose uptake in muscle in vivo by augmenting capillary recruitment. Diabetologia 2004;47:2226–34.
Bradley EA, Premilovac D, Betik AC, Hu D, Attrill E, Richards SM, et al. Metformin improves vascular and metabolic insulin action in insulin-resistant muscle. J Endocrinol. 2019;243:85–96.
Bradley EA, Zhang L, Genders AJ, Richards SM, Rattigan S, Keske MA. Enhancement of insulin-mediated rat muscle glucose uptake and microvascular perfusion by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. Cardiovasc Diabetol. 2015;14:91.
Sjoberg KA, Rattigan S, Jeppesen JF, Lundsgaard AM, Holst JJ, Kiens B. Differential effects of glucagon-like peptide-1 on microvascular recruitment and glucose metabolism in short- and long-term insulin resistance. J Physiol. 2015;593:2185–98.
Zhao L, Fu Z, Wu J, Aylor KW, Barrett EJ, Cao W, et al. Inflammation-induced microvascular insulin resistance is an early event in diet-induced obesity. Clin Sci (Lond). 2015;129:1025–36.
Rattigan S, Wallis MG, Youd JM, Clark MG. Exercise training improves insulin-mediated capillary recruitment in association with glucose uptake in rat hindlimb. Diabetes. 2001;50:2659–65.
Russell RD, Hu D, Greenaway T, Blackwood SJ, Dwyer RM, Sharman JE, et al. Skeletal Muscle Microvascular-Linked Improvements in Glycemic Control From Resistance Training in Individuals With Type 2 Diabetes. Diabetes Care. 2017;40:1256.
Durrer C, Lewis N, Wan Z, Ainslie PN, Jenkins NT, Little JP. Short-term low-carbohydrate high-fat diet in healthy young males renders the endothelium susceptible to hyperglycemia-induced damage, an exploratory analysis. Nutrients. 2019;11.
Morrison DJ, Kowalski GM, Bruce CR, Wadley GD. Modest changes to glycemic regulation are sufficient to maintain glucose fluxes in healthy young men following overfeeding with a habitual macronutrient composition. Am J Physiol Endocrinol Metab. 2019;316:E1061–70.
Parry SA, Turner MC, Woods RM, James LJ, Ferguson RA, Cocks M, et al. High-fat overfeeding impairs peripheral glucose metabolism and muscle microvascular eNOS Ser1177 phosphorylation. J Clin Endocrinol Metab. 2020;105:65–77.
Boon MR, Bakker LE, Haks MC, Quinten E, Schaart G, Van Beek L, et al. Short-term high-fat diet increases macrophage markers in skeletal muscle accompanied by impaired insulin signalling in healthy male subjects. Clin Sci (Lond). 2015;128:143–51.
Cahill F, Amini P, Wadden D, Khalili S, Randell E, Vasdev S, et al. Short-term overfeeding increases circulating adiponectin independent of obesity status. PLoS One. 2013;8:e74215.
Wadden D, Cahill F, Amini P, Randell E, Vasdev S, Yi Y, et al. Circulating glucagon-like peptide-1 increases in response to short-term overfeeding in men. Nutr Metab (Lond). 2013;10:33.
Boden G, Homko C, Barrero CA, Stein TP, Chen X, Cheung P, et al. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci Transl Med. 2015;7:304re7.
Brøns C, Jensen CB, Storgaard H, Hiscock NJ, White A, Appel JS, et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol. 2009;587:2387–97.
Bakker LE, van Schinkel LD, Guigas B, Streefland TC, Jonker JT, van Klinken JB, et al. A 5-day high-fat, high-calorie diet impairs insulin sensitivity in healthy, young South Asian men but not in Caucasian men. Diabetes 2014;63:248–58.
Adochio RL, Leitner JW, Gray K, Draznin B, Cornier MA. Early responses of insulin signaling to high-carbohydrate and high-fat overfeeding. Nutr Metab (Lond). 2009;6:37.
Anderson AS, Haynie KR, McMillan RP, Osterberg KL, Boutagy NE, Frisard MI, et al. Early skeletal muscle adaptations to short-term high-fat diet in humans before changes in insulin sensitivity. Obes (Silver Spring). 2015;23:720–4.
Dirlewanger M, di Vetta V, Guenat E, Battilana P, Seematter G, Schneiter P, et al. Effects of short-term carbohydrate or fat overfeeding on energy expenditure and plasma leptin concentrations in healthy female subjects. Int J Obes Relat Metab Disord. 2000;24:1413–8.
Emanuel AL, Meijer RI, Woerdeman J, van Raalte DH, Diamant M, Kramer MHH, et al. Effects of a hypercaloric and hypocaloric diet on insulin-induced microvascular recruitment, glucose uptake, and lipolysis in healthy lean men. Arterioscler Thromb Vasc Biol 2020;40:1695–704.
Wardle SL, Macnaughton LS, McGlory C, Witard OC, Dick JR, Whitfield PD, et al. Human skeletal muscle metabolic responses to 6 days of high-fat overfeeding are associated with dietary n-3PUFA content and muscle oxidative capacity. Physiol Rep. 2020;8:e14529.
McLaughlin T, Craig C, Liu LF, Perelman D, Allister C, Spielman D, et al. Adipose cell size and regional fat deposition as predictors of metabolic response to overfeeding in insulin-resistant and insulin-sensitive humans. Diabetes 2016;65:1245–54.
Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–28.
Wiedemann MS, Wueest S, Item F, Schoenle EJ, Konrad D. Adipose tissue inflammation contributes to short-term high-fat diet-induced hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2013;305:E388–95.
Andrich DE, Ou Y, Melbouci L, Leduc-Gaudet JP, Auclair N, Mercier J, et al. Altered lipid metabolism impairs skeletal muscle force in young rats submitted to a short-term high-fat diet. Front Physiol. 2018;9:1327.
Summers SA, Goodpaster BH. CrossTalk proposal: intramyocellular ceramide accumulation does modulate insulin resistance. J Physiol. 2016;594:3167–70.
Grundleger ML, Thenen SW. Decreased insulin binding, glucose transport, and glucose metabolism in soleus muscle of rats fed a high fat diet. Diabetes. 1982;31:232.
Bui C, Petrofsky J, Berk L, Shavlik D, Remigio W, Montgomery S. Acute effect of a single high-fat meal on forearm blood flow, blood pressure and heart rate in healthy male Asians and Caucasians: a pilot study. Southeast Asian J Trop Med Public Health. 2010;41:490–500.
Esser D, Oosterink E, Op ‘t Roodt J, Henry RM, Stehouwer CD, Muller M, et al. Vascular and inflammatory high fat meal responses in young healthy men; a discriminative role of IL-8 observed in a randomized trial. PLoS One. 2013;8:e53474.
Raitakari OT, Lai N, Griffiths K, McCredie R, Sullivan D, Celermajer DS. Enhanced peripheral vasodilation in humans after a fatty meal. J Am Coll Cardiol. 2000;36:417–22.
Keogh Jennifer B, Grieger Jessica A, Noakes M, Clifton Peter M. Flow-mediated dilatation is impaired by a high–saturated fat diet but not by a high-carbohydrate diet. Arterioscler Thromb Vasc Biol. 2005;25:1274–9.
Eggleston EM, Jahn LA, Barrett EJ. Early microvascular recruitment modulates subsequent insulin-mediated skeletal muscle glucose metabolism during lipid infusion. Diabetes Care. 2013;36:104–10.
Chai W, Liu J, Jahn LA, Fowler DE, Barrett EJ, Liu Z. Salsalate attenuates free fatty acid-induced microvascular and metabolic insulin resistance in humans. Diabetes Care. 2011;34:1634–8.
Clerk LH, Rattigan S, Clark MG. Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes. 2002;51:1138–45.
Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab. 2011;96:438–46.
Zhao L, Fu Z, Wu J, Aylor KW, Barrett EJ, Cao W, et al. Inflammation-induced microvascular insulin resistance is an early event in diet-induced obesity. Clin Sci (Lond, Engl: 1979). 2015;129:1025–36.
de Jongh RT, Serne EH, Ijzerman RG, de Vries G, Stehouwer CD. Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes. 2004;53:2873–82.
Shah DI, Singh M. Possible role of Akt to improve vascular endothelial dysfunction in diabetic and hyperhomocysteinemic rats. Mol Cell Biochem. 2007;295:65–74.
Chai W, Fu Z, Aylor KW, Barrett EJ, Liu Z. Liraglutide prevents microvascular insulin resistance and preserves muscle capillary density in high-fat diet-fed rats. Am J Physiol Endocrinol Metab. 2016;311:E640–8.
Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61:888–96.
Zhao L, Fu Z, Wu J, Aylor KW, Barrett EJ, Cao W, et al. Globular adiponectin ameliorates metabolic insulin resistance via AMPK-mediated restoration of microvascular insulin responses. J Physiol. 2015;593:4067–79.
Schmidt SL, Kealey EH, Horton TJ, VonKaenel S, Bessesen DH. The effects of short-term overfeeding on energy expenditure and nutrient oxidation in obesity-prone and obesity-resistant individuals. Int J Obes (Lond). 2013;37:1192–7.
Lundsgaard AM, Fritzen AM, Sjoberg KA, Kleinert M, Richter EA, Kiens B. Small amounts of dietary medium-chain fatty acids protect against insulin resistance during caloric excess in humans. Diabetes. 2021;70:91–8.
Whytock KL, Shepherd SO, Cocks M, Wagenmakers AJM, Strauss JAYoung. healthy males and females present cardiometabolic protection against the detrimental effects of a 7-day high-fat high-calorie diet. Eur J Nutr. 2021;60:1605–17.
Funding
Dr Lewan Parker was supported by a NHMRC & National Heart Foundation Early Career Fellowship (APP1157930).
Author information
Authors and Affiliations
Contributions
LC, MK and GK were responsible for the search of the articles for the review. LC and GK wrote the first draft of the manuscript. All authors made a significant intellectual contribution to the interpretation of studies and editing of several drafts of the manuscript. All authors have approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carmichael, L., Keske, M.A., Betik, A.C. et al. Is vascular insulin resistance an early step in diet-induced whole-body insulin resistance?. Nutr. Diabetes 12, 31 (2022). https://doi.org/10.1038/s41387-022-00209-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-022-00209-z