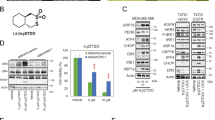
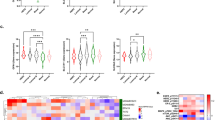
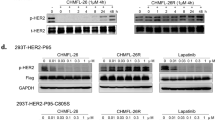
Abstract
While HER2 and EGFR are overexpressed in breast cancers and multiple other types of tumors, the use of EGFR and/or HER2 inhibitors have failed to cure many cancer patients, largely because cancers acquire resistance to HER2/EGFR-specific drugs. Cancers that overexpress the HER-family proteins EGFR, HER2, and HER3 are uniquely sensitive to agents that disrupt HER2 and EGFR protein folding. We previously showed that disruption of disulfide bond formation by Disulfide Disrupting Agents (DDAs) kills HER2/EGFR overexpressing cells through multiple mechanisms. Herein, we show that interference with proline isomerization in HER2/EGFR overexpressing cells also induces cancer cell death. The peptidyl-prolyl isomerase inhibitor Cyclosporine A (CsA) selectively kills EGFR+ or HER2+ breast cancer cells in vitro by activating caspase-dependent apoptotic pathways. Further, CsA synergizes with the DDA tcyDTDO to kill HER2/EGFR overexpressing cells in vitro and the two agents cooperate to kill HER2+ tumors in vivo. There is a critical need for novel strategies to target HER2+ and EGFR+ cancers that are resistant to currently available mechanism-based agents. Drugs that target HER2/EGFR protein folding, including DDAs and CsA, have the potential to kill cancers that overexpress EGFR or HER2 through the induction of proteostatic synthetic lethality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chlebowski RT, Manson JE, Anderson GL, Cauley JA, Aragaki AK, Stefanick ML, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105:526–35.
Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr., Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–16.
Wang M, Law ME, Castellano RK, Law BK. The unfolded protein response as a target for anticancer therapeutics. Crit Rev Oncol/Hematol. 2018;127:66–79.
Stocki P, Chapman DC, Beach LA, Williams DB. Depletion of cyclophilins B and C leads to dysregulation of endoplasmic reticulum redox homeostasis. J Biol Chem. 2014;289:23086–96.
Dunyak BM, Gestwicki JE. Peptidyl-proline isomerases (PPIases): targets for natural products and natural product-inspired compounds. J Med Chem. 2016;59:9622–44.
Theuerkorn M, Fischer G, Schiene-Fischer C. Prolyl cis/trans isomerase signalling pathways in cancer. Curr Opin Pharmacol. 2011;11:281–7.
Werneck MB, Hottz E, Bozza PT, Viola JP. Cyclosporin A inhibits colon cancer cell growth independently of the calcineurin pathway. Cell Cycle. 2012;11:3997–4008.
Ciechomska IA, Gabrusiewicz K, Szczepankiewicz AA, Kaminska B. Endoplasmic reticulum stress triggers autophagy in malignant glioma cells undergoing cyclosporine a-induced cell death. Oncogene. 2013;32:1518–29.
Robinson PJ, Pringle MA, Woolhead CA, Bulleid NJ. Folding of a single domain protein entering the endoplasmic reticulum precedes disulfide formation. J Biol Chem. 2017;292:6978–86.
Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–34.
Ogiso H, Ishitani R, Nureki O, Fukai S, Yamanaka M, Kim JH, et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002;110:775–87.
Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11:495–505.
Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr., et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–60.
Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science. 2002;297:1330–3.
Ferreira RB, Law ME, Jahn SC, Davis BJ, Heldermon CD, Reinhard M, et al. Novel agents that downregulate EGFR, HER2, and HER3 in parallel. Oncotarget. 2015;6:10445–59.
Ferreira RB, Wang M, Law ME, Davis BJ, Bartley AN, Higgins PJ, et al. Disulfide bond disrupting agents activate the unfolded protein response in EGFR- and HER2-positive breast tumor cells. Oncotarget. 2017;8:28971–89.
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–31.
Iyer RP, Egan W, Regan JB, Beaucage SL. 3h-1,2-Benzodithiole-3-One 1,1-Dioxide as an improved sulfurizing reagent in the solid-phase synthesis of oligodeoxyribonucleoside phosphorothioates. J Am Chem Soc. 1990;112:1253–4.
Srivastava PK, Field L. Organic disulfides and related substances .36. some oxodisulfide cleavage reactions to form disulfides and trisulfides. J Org Chem. 1972;37:4196–8.
Bass SW, Evans SA. C-13 Nuclear magnetic-resonance spectral properties of alkyl disulfides, thiolsulfinates, and thiolsulfonates. J Org Chem. 1980;45:710–5.
Goethals EJ, Huylebro J, Smolders W. Synthesis of thiosultones. B Soc Chim Belg. 1969;78:191–&.
Hartman DA. Determination of the stability of drugs in plasma. Curr Protoc Pharmacol. 2003;Unit 7:6. Chapter 7
Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6.
Shimizu T, Martin MS, Pelletier H, Lagadec P, Martin F. Effects of cyclosporin A on progressive and regressive tumors induced by two cancer lines derived from a single colon carcinoma chemically induced in the rat. Immunobiology. 1989;178:401–15.
DeRose YS, Gligorich KM, Wang G, Georgelas A, Bowman P, Courdy SJ, et al. Patient-derived models of human breast cancer: protocols for in vitro and in vivo applications in tumor biology and translational medicine. Curr Protoc Pharmacol. 2013;Unit14:23. Chapter 14
Neckers L. Effects of geldanamycin and other naturally occurring small molecule antagonists of heat shock protein 90 on HER2 protein expression. Breast Dis. 2000;11:49–59.
Takenokuchi M, Miyamoto K, Saigo K, Taniguchi T, Bortezomib Causes ER. Stress-related death of acute promyelocytic leukemia cells through excessive accumulation of PML-RARA. Anticancer Res. 2015;35:3307–16.
Anderson DJ, Le Moigne R, Djakovic S, Kumar B, Rice J, Wong S, et al. Targeting the AAA ATPase p97 as an approach to treat cancer through disruption of protein homeostasis. Cancer Cell. 2015;28:653–65.
Vekaria PH, Home T, Weir S, Schoenen FJ, Rao R. Targeting p97 to disrupt protein homeostasis in cancer. Front Oncol. 2016;6:181.
Kallioniemi OP, Kallioniemi A, Kurisu W, Thor A, Chen LC, Smith HS, et al. ERBB2 amplification in breast cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci USA. 1992;89:5321–5.
Field L, Khim YH. Organic disulfides and related substances. 33. sodium 4-(2-acetamidoethyldithio)butanesulfinate and related compounds as antiradiation drugs. J Med Chem. 1972;15:312–5.
Hysing J, Wist E. Cardiotoxic effects of trastuzumab. Tidsskr Nor Laege. 2011;131:2239–41.
Kopper L. Lapatinib: a sword with two edges. Pathol Oncol Res. 2008;14:1–8.
Erdogan E, Lamark T, Stallings-Mann M, Lee J, Pellecchia M, Thompson EA, et al. Aurothiomalate inhibits transformed growth by targeting the PB1 domain of protein kinase Ciota. J Biol Chem. 2006;281:28450–9.
Feng X, Holmlund T, Zheng C, Fadeel B. Proapoptotic effects of the novel proteasome inhibitor b-AP15 on multiple myeloma cells and natural killer cells. Exp Hematol. 2014;42:172–82.
Singh N, Joshi R, Komurov K. HER2-mTOR signaling-driven breast cancer cells require ER-associated degradation to survive. Sci Signal. 2015;8:ra52.
Ni S, Yuan Y, Huang J, Mao X, Lv M, Zhu J, et al. Discovering potent small molecule inhibitors of cyclophilin A using de novo drug design approach. J Med Chem. 2009;52:5295–8.
Ahmed-Belkacem A, Colliandre L, Ahnou N, Nevers Q, Gelin M, Bessin Y, et al. Fragment-based discovery of a new family of non-peptidic small-molecule cyclophilin inhibitors with potent antiviral activities. Nat Commun. 2016;7:12777.
Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–80.
Garg AD, Agostinis P. ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochem Photobiol Sci. 2014;13:474–87.
Law ME, Ferreira RB, Davis BJ, Higgins PJ, Kim JS, Castellano RK, et al. CUB domain-containing protein 1 and the epidermal growth factor receptor cooperate to induce cell detachment. Breast Cancer Res. 2016;18:80.
Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607.
Law BK, Waltner-Law ME, Entingh AJ, Chytil A, Aakre ME, Norgaard P, et al. Salicylate-induced growth arrest is associated with inhibition of p70s6k and down-regulation of c-myc, cyclin D1, cyclin A, and proliferating cell nuclear antigen. J Biol Chem. 2000;275:38261–7.
Acknowledgements
We thank breast cancer research advocate Mrs. Jeri Francoeur for her advice and support.
Funding
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Breast Cancer Research Program under Award Nos. W81XWH-15-1-0199 (BL) and W81XWH-15-1-0200 (RC). These studies were supported in part by grants from the Florida Breast Cancer Foundation (BL and RC), The Ocala Royal Dames for Cancer Research (BL), the Florida Department of Health Bankhead-Coley Program (4BF03, BL), the Collaboration of Scientists for Critical Research in Biomedicine (CSCRB, Inc., BL), and a UF-Health Cancer Center pilot project award (BL, RC, and CH). RF is grateful to the University of Florida for a Graduate School Fellowship.
Author contributions
MW, RF, ML, EY, AG, AS, BA, RC, and BL planned the studies. MW, RF, ML, BD, EY, AG, AS, and BL carried out the studies. BA, ER, CC, CH, and SN provided advice and reagents necessary for carrying out the studies. MW, RF, ML, EY, AG, AS, BA, ER, CC, SN, CH, RC, and BL edited the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, M., Ferreira, R.B., Law, M.E. et al. A novel proteotoxic combination therapy for EGFR+ and HER2+ cancers. Oncogene 38, 4264–4282 (2019). https://doi.org/10.1038/s41388-019-0717-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0717-6