Abstract
Background
Inherent to clinical research is the informed consent process, with the informed consent form (ICF), a key component of human participant protections. We wished to examine whether a shortened and simplified ICF, accompanied by an appendix, improved participant understanding of a study compared with a conventional ICF.
Methods
A shortened ICF was developed from an existing conventional ICF for a neonatal study. Either the shortened or conventional ICF was randomly distributed to members of two parental advocacy groups. Participants answered survey questions about the form they received.
Results
Thirty-one out of forty-one (76%) parents in the shortened ICF and 28/41 (68%) in the conventional ICF group responded. Significantly more parents in the shortened ICF group found their form “short and to the point”. Although they also stated that the shortened ICF did not provide enough information, there were no significant differences between groups measuring the understanding of key study components.
Conclusion
A shortened ICF did not impact the understanding of the clinical trial. It will be important to compare the shortened and conventional forms in actual clinical trials.
Similar content being viewed by others
Introduction
Participation in clinical research is necessary for the improvement of care delivered to patients. Inherent to the conduct of proper and ethical clinical research is the concept of informed consent. While the need for informed consent prior to participation in a research project is a legal requirement, there is an ethical and regulatory obligation to provide research participants or their proxies sufficient information regarding study details to ensure that their consent or permission is truly “informed”. The American Academy of Pediatrics (AAP) recommends that informed consent discloses information to patients and their surrogates and obtains legal authorization before undertaking any interventions.1
The ethical underpinnings of the informed consent process are autonomy and the protection from harm.2 Inherent to informed consent in children are the following key concepts: (1) the parent(s)/guardian (hereinafter parent) must have the capacity to decide that their child can participate in a research study, (2) there should be full disclosure of all relevant information, (3) the study information should be presented in the simplest possible terms to facilitate parental understanding, (4) the decision to participate must be voluntary, and (5) informed consent should be a process between the investigator and the parent(s) that is not limited to simply reading a consent form.3 Perhaps, the most important aspect of the informed consent process is the ability of a parent to understand what they are agreeing to. The informed consent process is especially difficult in vulnerable populations such as critically ill neonates and children.4 As medical care and research have become more complex, it can be difficult for a parent to determine if enrolling his or her child in a research study is in the best interest of the child. Decision-making may be even more challenging when the potential participant is critically ill and the emotional and contextual status of their surrogate may impact their decision-making capacity.
While an understanding of what is being consented to is important, previous studies have shown that researchers often fall short of developing understandable informed consent documents.5,6,7 Furthermore, over the past 20 years, the length of informed consent documents has nearly tripled.8 This is not due to enhanced explanations of the research question or procedures, but from additional institutional language relating to legal, ethical, insurance, and financial matters as well as technical details relating to data safety and storage.8 The median length for some informed consent documents has been shown to be greater than 22 pages, with consent forms for some vaccine trials more than 27 pages.9 For reasons that are unclear, forms used in the United States are ~10 pages longer than those used in international trials and forms used in adult trials are significantly longer than those used for pediatric trials.9 There is no evidence that these longer consent forms have led to improved understanding of study procedures, risks, or benefits or the protection and safety of research participants. In fact, they may actually compromise subject understanding of the presented material and reduce the number of research participants.10,11 The complexity of language in the consent form has also been a concern. The average readability of consent forms ranges between the 9th- and 12th-grade level, and pediatric consent forms can be more complex than adult forms.9 This is especially problematic as 50% of American adults cannot read at an 8th-grade level and 45 million Americans are functionally illiterate, reading below a 5th-grade level.12
The utility and potential advantage of a short consent form with an appendix has not been studied in the context of parents and surrogates of children who may be eligible for pediatric clinical trials. We believe this is necessary given pediatric participants represent a special population deserving of special protections from unacceptable amounts of potential risk. Given this, their surrogate’s understanding of presented information in an informed consent form (ICF) must be optimized. We hypothesized that a shortened ICF with an attached appendix would be equivalent to a conventional ICF in terms of the understanding of key elements of informed consent such as study procedures, potential risks and benefits, and the voluntary nature of participation and withdrawal. We also believed that parents would find the shortened consent form easier to read and understand.
Methods
Study design
We conducted a study of the adequacy/acceptability of a shortened ICF with an attached appendix compared with a conventional ICF presented to parents who were members of two different child health and research advocacy groups (Preemie Parent Alliance, International Children’s Advisory Network). This study was reviewed by the Institutional Review Board (IRB) at Tufts Medical Center.
Informed consent forms
The conventional ICF was previously used in a clinical trial of preterm neonates that was reviewed by the Food and Drug Administration (FDA) and approved by the IRB at Tufts Medical Center (NCT# 01941745). The form was 11-pages long and written according to the Tufts Health Sciences IRB guidelines. Of note, the trial was closed to enrollment at the time of this study which was communicated to the parents. The shortened ICF consisted of a two-page document that described the purpose of the study, study procedures, and the risks and benefits in a simple sentence structure. An appendix accompanied this document and contained additional legal language regarding research-related injury, protected health information, details on IRB and FDA reviews, and additional items required by the IRB. Both ICFs contained all the elements required by the IRB. The shortened ICF had a calculated Flesch Kincaid Grade Level of 7.2 versus 12.3 for the conventional ICF. The calculated Flesch Kincaid Grade Level for the shortened ICF appendix was 9.8. The word count of the shortened ICF was 792 words, while the word count for the conventional ICF was 4897. The appendix contained 3134 words and was written in a question-and-answer format as a reference for parents to review after reading the shortened ICF. For example, a topic heading depicted as, “What are some words that may be unfamiliar to me” was followed by bolded words with their associated definitions. Please see Box 1 for examples of language used in the shortened ICF and the attached appendix.
Assessment
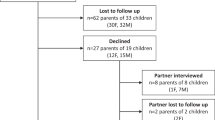
Both subjective and objective responses were solicited from respondents. The goal of enrollment was 90 subjects, 45 in the shortened ICF group, and 45 in the conventional ICF group. This number was selected in order to show a difference of at least 0.5 standard deviation of understanding (i.e., answering questions correctly) between the two groups with a target power of 0.8 and alpha of 0.05 addressing our primary hypothesis. Table 1 contains a list of subjective questions that were designed to assess the specifics of the consent form itself as well as information contained in the appendix. This was designed to assess whether the parent participants actually reviewed the appendix in detail. Objective questions were based on the validated Deaconess Informed Consent Comprehension Test and designed to assess the understanding of the key study components of the clinical trial (Table 2).13 The percentage of correct responses to the objective questions were used to determine differences between the two groups in terms of their understanding of key study-related components. Members of the Parent Preemie Alliance received either the shortened or conventional ICF and survey instrument via an email list-serve. Due to limitations of the email list-serve, only 41 parents’ email addresses were randomly selected by an administrator of the group. Forty-one members of the International Children’s Advisory Network received either the shortened or conventional ICFs in person at their annual meeting to attempt to keep the numbers in each group as similar as possible. Either the shortened ICF or the conventional ICF was placed at the parents’ tables prior to the start of the session. Parents were told that this was an assessment of understanding of the form they received. One author (P.D.M.) moderated the session to ensure that parents were not discussing the forms they received with one another. Parents who were present at the annual meeting and who participated in the assessment may or may not have had a child with a serious medical condition or previous experience with clinical research studies. In total, 82 parents were randomized to either the shortened ICF or the conventional ICF.
Answers were recorded using the Tufts Qualtrics (Provo, UT) online response system. Respondents in the Parent Preemie Alliance answered their surveys directly into the Tufts Qualtrics system. Respondents in the International Children’s Advisory Network gave their survey responses to the author who moderated their session. Their responses were then entered into the Tufts Qualtrics online response system. Differences between the two groups were calculated using the Real Statistics Microsoft Excel Function (Redmond, WA) Fischer’s exact test.
Results
Thirty-one out of forty-one parents (76%) who received the shortened ICF and 28/41 parents (68%) in the conventional form group completed the survey. There were no significant differences in objective-question responses, with 87% correct responses in the shortened ICF group and 89% in the conventional form group. Differences in subjective questions are found in Table 3. As expected, 27 of 31 (87%) parents in the shortened consent form group felt that the form was “short and to the point” compared with 17 of 28 parents (61%) reading the longer form (p < 0.05). However, significantly more parents reading the shortened consent form (35%) stated that the form did not provide them with sufficient information compared with the longer form (4%) even though the shortened form objectively contained the same information and did not impact the understanding of the research study (p < 0.05). Sufficient demographic information on the respondents was not provided and thus is not presented.
Discussion
In this pilot study, a shortened and simplified ICF was comparable to the conventional longer form in helping parents understand key research-related issues. The majority (87–89%) of responses in both the shortened ICF and the conventional ICF groups were answered correctly regarding study purpose, study risks, and potential benefits. This confirms our primary hypothesis that a shortened ICF with an attached appendix is equivalent to a longer form in educating parents about the research study. A shortened form may be helpful for recruitment as well, given that most research studies fail to meet recruitment goals, and the complexity of the informed consent process may contribute to a lack of research participation.14,15 A shortened and simplified form should lead to enhanced subject recruitment. The comparability of the two forms is similar to what has been reported in other studies, examining the utility of a shortened or simplified ICF.16,17
In prior studies, the concept of randomization was particularly challenging in the context of the ICF; in this study, the majority of respondents from both groups answered questions regarding randomization correctly.6 This may be due to the groups’ interest in research advocacy to improve outcomes or prior experience with a child in the hospital. Thus, they may not be naïve to the concept of randomization in research design.
The respondents demonstrated an interesting paradox in the subjective questions of the survey. While there were significantly more respondents who felt the information presented in the shortened ICF was “short and to the point” compared with the conventional form, those in the shortened ICF group also indicated that the shortened form “did not contain enough information.” This may be related to their experience with ICFs and a possible expectation of a more complex ICF, as opposed to the simplified and shortened ICF they received. Nevertheless, the subjective experience did not impact the ability to accurately answer questions testing comprehension. This indicates that shortening and simplifying the ICF did not compromise understanding.
The US Department of Health and Human Services (HHS) recently revised the Common Rule, a set of federal regulations for ethical conduct of human-subjects research.18 One of the central and most important changes was to the informed consent document, requiring that “key information” be presented that is most relevant to a person’s decision to participate in the study.18 Ideally, the consent documents should be concise and focused to better help a subject or their surrogate make an informed decision about their potential participation in a research project.19 The consent document is intended to foster communication between the researcher and potential participant and function as a reference document to which the participant can refer to in the future.20
A number of interventions have been attempted in an effort to enhance the understanding of ICFs, but results have been inconsistent. In one review, the use of an ICF associated with extended discussions was shown to be the only intervention that enhanced understanding.7 These extended discussions took place either via telephone or in person as supplementary interventions to the standard informed consent discussions.21,22 A recent Delphi methodological study has supported the use of a supplementary appendix to segregate those elements in the consent form that are not specific to the research, thereby decreasing the length of consent forms and improving understanding.23 A shortened form might address the concern that potential participants do not read the form and simply sign the ICF without adequate comprehension.20 A shortened ICF might also prompt enriched discussion between the investigator and participant, an approach that has affirmatively been shown to increase research participant understanding of study-related material.7 Randomly assigning participants to the use of a shortened ICF with an attached appendix and enriched follow-up discussions with a researcher versus a conventional form and standard informed consent process will be studied in the next phase of our studies.
There were several limitations to this study. First, this was a simulated experience: the parents did not have a critically ill neonate in the hospital and were not actually considering whether their child would benefit by participating in the study. This is consistent with the majority of randomized studies examining various interventions to improve the informed consent process that has been simulated.7 Second, the respondents were part of parental advocacy groups with specific interest in both prematurity and pediatric research. This selected group of parents were not naïve and may not represent the average parent of a child with respect to understanding of complex medical issues, including research-related risks and benefits. Third, the participants were told that they would be tested on the information presented to them, a warning that likely increased their attention to the ICF description of study-related procedures. While potential research participants are told of the importance of understanding the ICF material presented, they are not explicitly tested on the presented material. Whether the advance notice of a test on the material may serve to increase attention and understanding is a subject that should be studied by empirical analysis. Further, the role and utility of new types of electronic consent forms with “teach back” methodology to enhance understanding of the research process is also an important consideration that is amenable to study. Finally, given the sample size of the study and the power, small differences between the two groups may not have been detected.
The testing of a shortened ICF with an attached appendix in an active clinical trial, randomizing parents to receive either the shortened or conventional ICF, is needed to evaluate if this intervention can enhance the understanding of research-related procedures.21,22 Such a planned study will evaluate whether the difference in ICF length and complexity is important at a time of stress and emotional concern contemporaneous with caring for a sick child.
Conclusion
A shortened ICF with an attached appendix conveyed study-related information equally to a conventional ICF in this pilot study as measures by objective questioning. A third of responders receiving the shortened ICF stated that they did not “receive sufficient information” about the study, but this did not correlate with objective measures of comprehension. The shortened ICF and attached appendix is worthy of further investigation.
References
Katz, A. L. & Webb, S. A. Informed consent in decision making in pediatric practice. Pediatrics 138, e1–e16 (2001).
Jefford, M. & Moore, R. Improvement of informed consent and the quality of consent documents. Lancet Oncol. 9, 485–493 (2008).
Beauchamp, T. L. & Childress, J. F. Principles of Biomedical Ethics. 7th edn, (Oxford University Press, New York, 2012).
Menon, K. et al. Factors affecting consent in pediatric critical care research. Intensive Care Med. 38, 153–159 (2012).
Pick, A., Berry, S., Gilbert, K. & McCaul, J. Informed consent in clinical research. Nurs. Stand. 27, 44–47 (2013).
Falagas, M. E., Korbila, I. P., Giannopoulou, K. P. & Peppas, G. Informed consent: how much and what to patients understand? Am. J. Surg. 198, 420–435 (2009).
Nishimura, A. et al. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC Med. Ethics 14, 28–43 (2013).
Berger, O., Gronberg, B. H., Sand, K., Kaasa, S. & Loge, J. H. The length of consent documents in oncological trials is doubled in twenty years. Ann. Oncol. 20, 379–385 (2009).
Kass, N. E., Chaisson, L., Taylor, H. A. & Lohse, J. Length and complexity of US and international HIV consent forms from federal HIV network trials. J. Gen. Intern. Med. 26, 1324–1328 (2011).
Epstein, L. C. & Lasagna, L. Obtaining informed consent: form or substance? Arch. Int. Med. 123, 682–688 (1969).
Beardsley, E., Jefford, M. & Mileshkin, L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening? J. Clin. Oncol. 25, e13–e14 (2007).
Kirsch, I. S., Jungeblut, A., Jenkins, L., & Kolstad, A. Adult Literacy in America: A First Look at the Findings of the National Adult Literacy Survey. 3rd edn, (National Center for Education Statistics, US Department of Education, Office of Research and Improvement, Washington DC, 2002.
Miller, C. K., O’Donnell, D. C., Searight, R. & Barbarash, R. The Deaconess Informed Consent Comprehension Test: an assessment tool for clinical research subjects. Pharmacotherapy 16, 872–878 (1996).
Stein, M. A. et al. Research START: a multimethod study of barriers and accelerators of recruiting research participants. Clin. Transl. Sci. 8, 647–654 (2015).
Marco, C. A. Impact of detailed informed consent on research subjects’ participation: a prospective, randomized trial. J. Emerg. Med. 34, 269–275 (2008).
Coyne, C. A. et al. Randomized, controlled trial of an easy-to-read informed consent statement for clinical trial participation: a study of the Eastern Cooperative Oncology Group. J. Clin. Oncol. 21, 836–842 (2003).
Grady, C. et al. A randomized trial comparing concise and standard consent forms in the START trial. PLoS ONE 12, 1–16 (2017).
Federal Policy for the Protection of Human Subjects, Federal Register, https://www.federalregister.gov/documents/2015/09/08/2015-21756/federal-policy-for-the-protection-of-human-subjects. (9/8/2015). Washington, DC, cited 9/15/17 1213PM
Menikoff, J., Kaneshiro, J. & Pritchard, I. The common rule, updated. N. Engl. J. Med. 376, 613–615 (2017).
Lorell, B. H., Mikita, J. S., Anderson, A., Hallinan, Z. P. & Forrest, A. Informed consent in clinical research: consensus recommendation for reform identified by an expert interview panel. Clin. Trial 12, 692–693 (2015).
Freer, Y., McIntosh, N., Teunisse, S., Anand, K. J. & Boyle, E. M. More information, less understanding: a randomized study on consent issues in neonatal research. Pediatrics 123, 1301–1305 (2009).
Aaronson, N. K. et al. Telephone-based nursing intervention improves the effectiveness of the informed consent process in cancer clinical trials. J. Clin. Oncol. 14, 984–996 (1996).
Corneli, A. et al. Evidence-based strategies for shortening informed consent forms in clinical research. J Empir. Res. Hum. Res. Ethics 12, 14–25 (2017).
Acknowledgements
The authors wish to thank Lynn Hudson from the Critical Path Institute, as well as the leadership and members from the Preemie Parent Alliance and the International Children’s Advisory Network for their assistance with this project. This study was supported in part by the Clinical and Translational Science Award (CTSA) program from the National Center for Advancing Translational Sciences (NCATS) to Tufts University (UL1TR001064) and Harvard University (UL1 TR001102) and a grant from the FDA Office of Orphan Products Development R01 FD003899.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murray, P.D., Bierer, B.E., Hirschfeld, S. et al. Assessment of a shortened informed consent form for pediatric research: a pilot study. Pediatr Res 84, 516–519 (2018). https://doi.org/10.1038/s41390-018-0043-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0043-7
This article is cited by
-
Too Dense and Too Detailed: Evaluation of the Health Literacy Attributes of an Informed Consent Document
Journal of Racial and Ethnic Health Disparities (2020)
-
Facilitating Informed Permission/Assent/Consent in Pediatric Clinical Trials
Pediatric Drugs (2019)
-
Rethinking informed consent in pediatric research: a time for regulatory policy change?
Pediatric Research (2018)