Abstract
Diets rich in saturated fats have become a staple globally. Fifty percent of women of childbearing age in the United States are obese or overweight, with diet being a significant contributor. There is increasing evidence of the impact of maternal high-fat diet on the offspring microbiome. Alterations of the neonatal microbiome have been shown to be associated with multiple morbidities, including the development of necrotizing enterocolitis, atopy, asthma, metabolic dysfunction, and hypertension among others. This review provides an overview of the recent studies and mechanisms being examined on how maternal diet can alter the immune response and microbiome in offspring and the implications for directed public health initiatives for women of childbearing age.
Impact
-
Maternal diet is important in shaping the offspring microbiome and neonatal immune system.
-
Reviews the current literature in the field and suggests potential mechanisms and areas of research to be targeted.
-
Highlights the current scope of our knowledge of ideal nutrition during pregnancy and consideration for enhanced public health initiatives to promote well-being of the future generation.
Similar content being viewed by others
Introduction
The period of gestation for humans is marked by the transformation of a single cell into over a hundred trillion cells that amalgamate into a functional, metabolically active organism, capable of perpetuating the species. This requires a symphony of processes to function efficiently, and of essence to the success of procreation is the delivery of adequate nutrition to fuel the development of the being. The deliverance of nutrition to the fetus is complex and involves not just the maternal acquisition of food but also the successful breakdown, transfer to, and utilization by the fetus. The fetus is exposed to an array of various vitamins, minerals, metabolites, proteins, lipids, sugars, and xenobiotics that are a result of maternal choice. The nutrients are further modified by microbial metabolism in the maternal gastrointestinal track.
The impact of the quality of the maternal diet on offspring is therefore extremely important to understand. The landscape of nutrient availability, quality, and consumption has changed over the decades and in present time still varies depending on geographical location, socioeconomic status, environmental impact, media, and even economics. Data from the Pew Research Center show in 2010, the average American obtained close to 50% of their calories from refined carbohydrates and fats, which was an increase of 9.3% compared to 1970. The nutrients delivered to the fetus as a result of maternal choice and availability are not only important for the development of the organism but also permit sampling of the environment and can potentially influence the development of critical pathways to ensure successful survival. These pathways include maximization of metabolic processes, modulation of vascular tone, optimization of neurological connections, and balancing of the immune response. The subtleties of the quality of nutrition in affecting development of the fetus have historically been compelled into context by extremes of the result. The development of neural tube defects caused by maternal deficiency of folic acid, the loss of limbs with in utero exposure to Thalidomide, and the findings of mental retardation and altered facies in offspring with excessive maternal consumption of alcohol are a few examples.
Activation of the immune system is critical for survival of the species against pathogens and concurrent to this goal is the attenuation of the response to prevent unsolicited stimulation by wonted nutritional, environmental, and microbial exposures. Development of the immune system begins in utero and is dependent on appropriate nutrition.1 The microbiome is critical for the development and attenuation of the immune response after birth.2 The role of the maternal microbiome has recently been postulated to affect development of the offspring’s microbiome and immune response. Indeed, early colonization patterns in infants can also have an impact on the development of diseases in adulthood.3 The factors that alter the maternal microbiome during pregnancy and consequently affect offspring can give us insight into potential mechanisms and guide development of targeted interventions during this critical window.
The effect of diet on the maternal microbiome, and consequently on the infant microbiome and development, is potentially the most conducive to intervention. Women are more likely to seek medical care and change behaviors during pregnancy. Diet can alter the microbiome and therefore nutrition during pregnancy would likely be crucial to development of the offspring’s microbiome and immune development. Current guidelines for nutrition during pregnancy by American College of Obstetrics and Gynocology (ACOG), United States Dietary Association (USDA), and other agencies are focused on vitamins and micronutrients. The guidelines on macronutrient consumption are focused on total caloric intake depending on body mass index (BMI), the appropriate consumption of protein and essential fats, and adequate intake of carbohydrates.4 Indeed, most women in America consume an excess of recommended fat and carbohydrates daily and enhanced public health initiatives with focused dietary guidelines during pregnancy emphasizing benefit could have a large impact on pregnant women and their offspring.
This review will focus on our current understanding of the effect of macronutrient composition of the maternal diet on the microbiota of the infant and the link to immune development in utero. While obesity can affect programming of offspring, we will focus on studies related to maternal diet, since obesity is multifactorial and the goal is to determine if diet quality alone can have an impact on offspring. We will then illustrate the potential mechanisms for modulation of the fetal immune response and postnatal colonization by maternal diet with a focus on highlighting areas for future research and implications for potential public health initiatives for diet recommendations and development of nutritional therapeutics during pregnancy.
Evidence from human studies: effect of maternal diet on the infant microbiome and immune development
Human studies investigating the role of maternal nutrition on offspring are largely limited to the identification of associations rather than mechanisms. Feeding studies are difficult to do in a cohort of pregnant women. The majority of studies from humans methodologically utilizes food/diet questionnaires that are self-reported. They do however give us important clues on the potential impact of maternal diet on offspring.
Role of maternal diet on the neonatal/infant microbiome
Due to the high baseline consumption of fat in developed countries, many studies focus on dietary intake of saturated and polyunsaturated fats specifically. We identified two studies that investigate how maternal diet affects the neonatal microbiome. In a study utilizing a questionnaire to determine fat intake in the month prior to delivery, Chu et al.5 found that the infant gut microbiome varied in association with higher fat intake, and showed depletion of the beneficial genus of Bacteroides in neonates that persisted to 6 weeks of age.
In the New Hampshire Birth Cohort Study, Hoen and co-workers6 analyzed the association of maternal diet during pregnancy with the infant gut microbiome at 6 weeks post-delivery utilizing 145 mother–infant dyads. This study used a validated food frequency questionnaire that was administered during a limited period of time during pregnancy, specifically during 24–28 weeks gestation. They found a specific role for fruits and dairy consumption in altering the clustering of infants to specific microbial compositions, and clustering also varied depending on mode of delivery.
These studies show that maternal diet has an effect on the neonatal microbiome; however, the studies assess nutrition at different phases of pregnancy, second trimester vs end of third trimester. The timing of folic acid deficiency and subsequent development of neural tube defects is crucial, with deficiency in the first trimester being most harmful. Studies that determine dietary intake at all phases of pregnancy are necessary to determine how early vs late dietary practices alter the neonatal microbiome.
Maternal microbiome effect on infant microbiome
Several studies have already demonstrated an association between maternal diet on alteration of the maternal microbiome;7 but does the maternal microbiome influence development of neonatal and infant colonization? Associations linking the maternal microbiome, delivery mode, and postnatal feeding with neonatal colonization have been described.8,9,10 Studies that investigate bacterial communities at the strain level with higher resolution at the genomic level are more effective at tracking bacterial transmission from mother to offspring, and we discuss two of these studies here. Ferretti et al.11 longitudinally followed 25 mother–infant pairs and sampled the microbiome from multiple body sites for up to 4 months postpartum. They found that infants were colonized with maternal skin and vaginal strains only transiently and maternal gut strains persisted in the infant gut through 4 months of age. Of note, only 37–61% of the species in the infant at any given time point were also present in at least one body site from the mother, with other species likely having been acquired from the environment and other individuals over time. They found that strains in common between the mother and the infant decreased over time; this could be explained by the resilience of those bacterial strains or perhaps alternatively by the infant immune response to selection of strains.
Another study confirmed that vertical transmission of bacteria from the mother is important in the colonization of the infant microbiome, with some showing persistence up to 1 year old, but that mode of delivery strikingly altered composition of the infant microbiome.12 This study also showed that the similarity over time to the mother’s microbiota was not significantly higher than the similarity to unrelated mothers, suggesting that similarities are dependent on the child’s intestinal environment and diet, and not dominated by maternal transmission.
We can conclude from these studies that the maternal microbiome is important for the development of the neonatal and infant microbiome, and since maternal diet is important in shaping maternal gut bacterial composition, it is also implied that diet affects the neonatal and infant landscape. We can also conclude that simple longitudinal transfer does not completely explain the pattern of colonization in newborns and infants, and that other than acquisition from the environment and others, the intestine may be primed prior to delivery or after birth for tolerance to distinct bacterial communities.
Role of maternal diet and/or microbiome on infant immunity
The majority of studies on humans on the effect of maternal diet or the maternal microbiome on infant immunity is related to the outcome of development of eczema, allergies, or wheezing in early childhood. These conditions are largely related to the adaptive immune response; however, the innate immune response is important in regulating this response as well.13 We will discuss a selection of these studies here.
In a translational study, Peter and co-workers14 prospectively examined the bacterial colonization in pregnant women with inflammatory bowel disease (IBD) and assessed the effect on the composition on the offspring’s microbiome. They found that pregnant women with IBD had less bacterial diversity and altered composition compared to control women, and further that the offspring had the same features. Specifically, infants born to mothers with IBD had an increase in Gammaproteobacteria and a depletion in Bifidobacteria. They then neo-colonized germ-free mice with the human infant stool at 3 months of age. Indeed, they found that neo-colonization with IBD offspring microbiota resulted in fewer class-switched memory B cells and regulatory T cells in the colon of the mice. This study suggests that altered infant colonization due to maternal microbiota can affect the adaptive immune response in offspring.
A German prospective birth cohort study15 analyzed the association of diet during the last 4 weeks of pregnancy with the development of eczema and allergy in 2-year-old offspring. They showed that high intake of vegetable oils and margarine was associated with the development of eczema. Conversely, they found that high maternal fish intake was inversely associated with the development of eczema in 2-year-old offspring. Several other studies have found similar findings, including Calvani et al.,16 Romieu et al.,17 and others.18,19 Interestingly, a study which objectively evaluated maternal n-3 polyunsaturated fatty acid (PUFA) by determining n-3 PUFA levels on erythrocyte membranes found that high n-3 PUFA content was associated with differential patterns of methylation in the cord white blood cells of offspring,19 suggesting a lasting epigenetic effect on the immune cells. Another large prospective study from Japan,20 The Osaka Maternal and Child Health Study, found that higher intake of n-6 PUFAs of linoleic acid during pregnancy was independently related to an increase risk of eczema in infants aged 16–24 months, but not to the development of wheeze. They did not find an association with the consumption of n-3 PUFAs and protection from allergy.
These studies support the hypothesis that maternal diet and/or the microbiome can modulate the adaptive immune response. The studies are limited and do not evaluate longer term outcomes of the immune system, including the development of autoimmunity, susceptibility to infection, and other chronic illnesses. Further these studies are focused on the fat composition of diets, and support that the quality of fat, saturated versus polyunsaturated, and N-3 vs N-6, are important. High carbohydrate diets with refined carbohydrates have not been evaluated extensively and studies focused on fat, carbohydrate, and protein intake are needed. The non-human studies discussed next give us additional evidence for the effect of maternal diet on the infant microbiome and immunity, and provide some clues of the mechanisms involved.
Evidence from non-human studies: effect of maternal diet on the infant microbiome and immune development
Role of maternal diet on the neonatal/infant microbiome
The majority of non-human maternal diet models have focused on the effect of high-fat diet on the offspring microbiome. Utilizing a non-human primate model, specifically with Japanese macaques, Ma et al.21 investigated the impact of maternal high-fat diet (primarily saturated) versus low-fat diet on the microbiome of offspring. Female dams were maintained on an isocaloric diet consisting of either 36% fat or control chow, with 13% fat. A proportion of the dams remained lean, while some progressed to obesity, but despite this, the microbiota of the high-fat-diet mothers clustered together and were distinct from control dams. They then examined the offspring after weaning and found they had an altered gut microbiome that persisted over time despite being given a control diet post-weaning. Specifically, they found that Campylobacter, a commensal in Japanese macaques, was persistently diminished in the high-fat diet offspring.
Several studies in rodents have similarly found that maternal high-fat diet (40–60% fat, primarily saturated) alters the offspring gut microbiome as early as during the neonatal period22 and persists at or after weaning,23,24,25 despite offspring being weaned to a control diet. These studies also found that offspring weaned to a high-fat diet develop worse outcomes in disease models tested.
Besides high-fat modifications, alternative macronutrient diet studies are limited, but the results are intriguing. Li et al.26 compared the offspring gut microbiome of sows on low- and high-fiber diets. They found that piglets had unique microbial communities in the high-fiber diet when compared with the low-fiber diet. In a similar study on sows, Peng and co-workers27 found that increased soluble fiber diet in pregnant mothers altered the offspring microbiome. Soluble fibers can be degraded to short-chain fatty acids (SCFA) which have been shown to be important in regulation of the innate and adaptive immune responses.28,29
Maternal microbiome effect on infant immunity
As mentioned previously, studies have demonstrated the effect of maternal diet on the maternal microbiome.7 But what is the role of the maternal microbiome specifically during pregnancy on development of the offspring immune response? Characterization of germ-free mice demonstrate that immune development is dependent on bacterial colonization.30 Bacteria-naïve mice have less cellularity of intestinal immune organs, a reduction of T cells, reduction of secretory IgA, Paneth cells, and intestinal epithelial cells when compared to conventional mice.
An elegant study by Macpherson and co-workers31 examined the role of maternal colonization by bacteria on in utero development of the offspring innate immune system by transiently colonizing pregnant female mice. The study design utilized germ-free mice that were colonized with an Escherichia coli strain that cannot persist in the intestine; therefore, colonized mice become sterile again prior to delivery, obviating the effect of exposure to bacteria during delivery. The design allowed for insight into the role of bacterial colonization during gestation on offspring.
The authors found that transient gestational colonization with this single bacterial species during pregnancy increased the number of type 3 innate lymphoid cells (ILC3) and F4/80 mononuclear cells in the offspring intestine. This effect was persistent with effects seen up to 8 weeks of life in offspring, akin to adulthood. Further, they found that offspring born to colonized pregnant dams had altered intestinal transcriptional profiles and increased expression of epithelial antibacterial peptides compared to germ-free dams. They did not find live bacteria in the placenta in treated mice and concluded that the effects were likely from microbial products that presented to the fetus via maternal tissue. They further found that this effect was partially dependent on maternal IgG transfer to the fetus and exposure of the fetus to microbial molecules.
Diet can alter the composition of bacteria in the intestine in as little as 1–2 days.32,33 This study highlights how transient bacterial colonization during pregnancy with a specific strain affects development of the immune system of the fetus with effects lasting through to adulthood in mice. The potential for the effects of maternal diet on the immune response in offspring cannot be understated.
Maternal diet effect on infant immunity
Several rodent studies utilizing a maternal high-fat diet model have also demonstrated an effect on infant immunity. Datta and co-workers23 examined the effect of 40% high-fat diet (primarily saturated fat) on weaned offspring susceptibility to E. coli sepsis, Staphylococcus aureus infections, experimental autoimmune encephalitis, and anaphylaxis. They found that maternal high-fat diet offspring had increased disease susceptibility in all the models evaluated and that the effect was microbiota-dependent. This effect was not fully recapitulated in offspring born from control mothers who were fed a high-fat diet after weaning, suggesting a direct effect from exposure to the in utero maternal diet.
Other studies utilizing maternal high-fat diet models in mice have shown an effect on development of offspring ILC3 and susceptibility to necrotizing enterocolitis,22 gut barrier function and integrity,34 susceptibility to a model of IBD,25 epigenetic effects and increased risk for development of obesity, and diabetes.35
Thorburn et al.36 examined the role of a maternal high-fiber diet on offspring susceptibility to development of allergic airway disease (AAD) and found that high-fiber protected mouse offspring from AAD, potentially mediated by alteration of the maternal microbiome and increased SCFA. Hansen et al.37 found that maternal gluten-free diet resulted in a unique offspring microbiome in non-obese diabetic mice and protected from susceptibility to develop type 1 diabetes mellitus.
Taken together, these limited studies in humans and animal models provide some evidence for an independent role of maternal diet quality on development of the offspring microbiome and immunity. The strongest evidence is for the role of maternal high-fat diet and it is important to note that the quality of the fat is crucial, with the majority of models utilizing chow with saturated fats. Overall, the specific mechanisms are minimally explored and the impact of other macronutrient modifications on offspring remains relatively unknown. With alternate diets becoming more common, including ketogenic, low carbohydrate, Mediterranean, and gluten-free diets, further studies are needed. In the next section, we outline the potential mechanisms from current studies and highlight areas that warrant further exploration.
Potential mechanisms that underlie maternal diet-mediated effects on the infant microbiome and immunity
This review highlights that the quality of nutrition plays an important role in shaping the microbiota of the mother. In addition, maternal diet can alter the development of the offspring microbiome and the offspring immune response, with an effect that may persist into adulthood. There are likely several mechanisms for the effect of the maternal diet on offspring, which are not mutually exclusive. Here we will briefly explore the mechanisms and any studies that support the hypotheses:
-
(a)
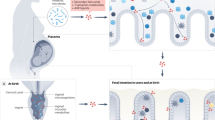
Maternal diet alters the maternal microbiome and results in altered in utero colonization and/or colonization in offspring at birth (Fig. 1a). There is still some controversy regarding in utero colonization;38,39 however, bacterial signatures have been found in the placenta,40 the fetal tissues,41 amniotic fluid,42 and the fetal intestine.43 The source of the bacteria could be from transfer from the maternal gastrointestinal (GI) tract or plasma. Diet can also affect the maternal microbiome in the GI tract, vagina, and skin, resulting in altered colonization at birth as the fetus passes through the birth canal or is born via C-section.
Fig. 1: Potential mechanisms for maternal diet-mediated effects on the fetus affecting postnatal colonization and immunity. a Maternal diet may modify the maternal microbiome resulting in altered exposure to live bacteria in utero and at birth via the vaginal canal or skin. b Maternal diet may alter the microbial processing of macro- and micronutrients and microbial colonization resulting in differential exposure in utero to microbial products, immunoglobulins, and cytokines. c Maternal diet can modulate the amount of beneficial and harmful dietary metabolites and dietary TLR ligands which the fetus becomes exposed to in utero. d Potential ways to maximize benefit for offspring microbiota and immune development include enhanced dietary guidelines for gravid women, probiotic supplementation, and nutraceutical supplementation either pre- or post-natally.
-
(b)
Maternal diet alters the presence of the maternal microbiome and transfer of microbial products, immunoglobulins, and cytokines to differentially prime the fetus for colonization and immunity (Fig. 1b). Recent data have questioned the existence of the placental microbiome39 and in utero colonization, but bacterial products may cross the placenta resulting in detection by sequencing without viability. These microbial products could alter Toll-like receptor (TLR) signaling in the fetal intestine44 and subsequently alter development of innate and adaptive immunity. They may be carried by maternal immunoglobulins in serum31 and cross the placenta into the fetus, preferentially priming the intestine for colonization. The fetus is continuously swallowing amniotic fluid, and the presence of cytokines and other growth factors in the amniotic fluid45 may be altered by maternal diet and consequently also impact the development of fetal gut immunity.46
-
(c)
Maternal diet can alter the presence of dietary metabolites and substrates in the placenta, amniotic fluid, or fetal tissues that differentially prime the fetus for colonization and immunity (Fig. 1c). Dietary TLR ligands47,48 and inhibitors49 may transfer via the placenta or be present in the amniotic fluid, allowing the fetal intestine to sample these compounds differentially. Other dietary ligands, metabolites,50 and endotoxins51 can also be present in fetal tissues to differentially prime the fetus in utero,52 including aryl hydrocarbons receptor ligands31 and SCFA.42
Research in this field has increased and further work aimed at identifying the mechanisms of modulation of offspring microbiota and immunity by maternal diet is needed, including the timing of intervention and changes in diet. This would allow for the development of improved recommendations for pregnant mothers, pre- and post- natal nutraceuticals, beneficial microbial supplements, and pharmacologic therapies to potentially maximize the health of both the mother and infant (Fig. 1d).
Implications for public health initiatives: what is the ideal diet during pregnancy?
The short answer is we still do not know. Current recommendations from the ACOG regarding pregnancy diet counseling4,53 includes screening the diet and vitamin supplements to confirm women are obtaining the recommended daily allowances, as well as to encourage attaining a BMI in the normal range. Weight gain during pregnancy is recommended based on prenatal BMI. The Guidelines for Perinatal Care published jointly by the ACOG and the American Association of Pediatrics in 2012 recommends advising women to consume “a balanced diet with the appropriate distribution of the basic food pyramid groups”. The dietary recommendations by the Office of Disease Prevention and Health Promotion as well as the USDA advise increased fruit and vegetable consumption and reduced sugar and saturated fat intake for individuals without discriminating between pregnant and non-pregnant women. The USDA and Department of Health & Human Services dietary interviews, What we eat in America (WWEIA), show that the typical female of childbearing age from 2015 to 2016 consumed an average of 1850 calories, of which 15.8% was from protein (221.3 g/day), 47.8% was from carbohydrates (73.1 g/day), and 36.4% was from fat (74.8 g/day) (Fig. 2). Over 40% of carbohydrate intake was from sugars and only 6.7% was from dietary fiber. In total, 32.7% of fat intake was from saturated fats. The ACOG recommends utilizing the USDA published website, choosemyplate.gov, to maximize appropriate diet choices. The USDA recommendations for pregnant women focuses on choices that limit calories from added sugars and saturated fats, as well as encouraging intake of omega-3 fats. Specific recommendations on macronutrients are generally varied (Fig. 2), except for the recommendation of at least 71 g of protein per day or 20–25% of intake per day54 and the encouragement of foods with omega-3 fats.
Data compiled from the USDA and DHHS dietary intake interviews conducted yearly “What we eat in America” (WWEIA) with average protein, carbohydrate, and fat intake in women aged between 19 and 50 years (green bars) between 2015 and 2016. Red floating bars are recommendation ranges of macronutrient consumption from the ACOG, USDA, and Office of Disease Prevention and Health Promotion.
The limited studies presented would suggest that maternal high-fat diet containing primarily saturated fats, in the absence of obesity, may have detrimental effects on the fetus. Further there is some evidence for the role of increased maternal dietary fiber intake in benefitting offspring. Whether or not pregnant women are obese, there is evidence to support the recommendation to reduce fat intake, particularly of saturated fats, during pregnancy and increase intake of dietary fiber. Public health initiatives that encourage pre-pregnancy and pregnancy counseling to include nutrition counseling and evaluation by a nutritionist could have a significant impact on the long-term health of offspring. In this tech savvy world, development and encouraging utilization of mobile and online applications to track and advise on food choices during pregnancy could be effective. Women during gestation should be encouraged that making alternate dietary decisions during pregnancy can yield tangible results for themselves and their child. Indeed, in a non-human primate model, switching obese mothers to a healthy diet demonstrated benefit,55 and women should be encouraged that dietary changes made during pregnancy are not futile.
Conclusion
It is encouraging that the studies discussed here support the role of maternal diet affecting the offspring microbiome and immunity, primarily because maternal diet is modifiable with the potential of lifelong benefit to the offspring. There is still a lack of clarity on the amount of macronutrients that are optimal during pregnancy, the effect of diet on different stages of gestation, the role of the quality of carbohydrates and protein on the maternal/neonatal microbiota, and the mechanisms that are responsible to maternal diet-mediated effects on offspring immunity. Further work in humans and animal models is needed with the goal of developing optimized guidelines for nutrition during pregnancy and development of non-pharmacologic supplements that can maximize the health outcomes of the future generation.
References
Obanewa, O. & Newell, M. L. Maternal nutritional status during pregnancy and infant immune response to routine childhood vaccinations. Future Virol. 12, 525–536 (2017).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Milani, C. et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 81, https://doi.org/10.1128/MMBR.00036-17 (2017).
Kominiarek, M. A. & Rajan, P. Nutrition recommendations in pregnancy and lactation. Med. Clin. North Am. 100, 1199–1215 (2016).
Chu, D. M. et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 8, 77 (2016).
Lundgren, S. N. et al. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 6, 109 (2018).
Maher, S. E. et al. The association between the maternal diet and the maternal and infant gut microbiome: a systematic review. Br. J. Nutr. 1–29, https://doi.org/10.1017/S0007114520000847 (2020).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Backhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015).
Bokulich, N. A. et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 8, 343ra382 (2016).
Ferretti, P. et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24, 133–145.e135 (2018).
Korpela, K. et al. Selective maternal seeding and environment shape the human gut microbiome. Genome Res. 28, 561–568 (2018).
Iwasaki, A. & Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16, 343–353 (2015).
Torres, J. et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut 69, 42–51 (2020).
Sausenthaler, S. et al. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am. J. Clin. Nutr. 85, 530–537 (2007).
Calvani, M. et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr. Allergy Immunol. 17, 94–102 (2006).
Romieu, I. et al. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin. Exp. Allergy. 37, 518–525 (2007).
Vuillermin, P. J. et al. The maternal microbiome during pregnancy and allergic disease in the offspring. Semin. Immunopathol. 39, 669–675 (2017).
Bianchi, M. et al. Maternal intake of n-3 polyunsaturated fatty acids during pregnancy is associated with differential methylation profiles in cord blood white cells. Front. Genet. 10, 1050 (2019).
Miyake, Y., Sasaki, S., Tanaka, K., Ohfuji, S. & Hirota, Y. Maternal fat consumption during pregnancy and risk of wheeze and eczema in Japanese infants aged 16-24 months: the Osaka Maternal and Child Health Study. Thorax 64, 815–821 (2009).
Ma, J. et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat. Commun. 5, 3889 (2014).
Babu, S. T. et al. Maternal high-fat diet results in microbiota-dependent expansion of ILC3s in mice offspring. JCI insight 3, https://doi.org/10.1172/jci.insight.99223 (2018).
Myles, I. A. et al. Parental dietary fat intake alters offspring microbiome and immunity. J. Immunol. 191, 3200–3209 (2013).
Bhagavata Srinivasan, S. P., Raipuria, M., Bahari, H., Kaakoush, N. O. & Morris, M. J. Impacts of diet and exercise on maternal gut microbiota are transferred to offspring. Front. Endocrinol. 9, 716 (2018).
Xie, R. et al. Maternal high fat diet alters gut microbiota of offspring and exacerbates DSS-induced colitis in adulthood. Front. Immunol. 9, 2608 (2018).
Li, Y. et al. Maternal dietary fiber composition during gestation induces changes in offspring antioxidative capacity, inflammatory response, and gut microbiota in a sow model. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21010031 (2019).
Cheng, C. et al. Maternal soluble fiber diet during pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets. Appl. Environ. Microbiol. 84, https://doi.org/10.1128/AEM.01047-18 (2018).
Goncalves, P., Araujo, J. R. & Di Santo, J. P. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm. Bowel Dis. 24, 558–572 (2018).
Needell, J. C. et al. Maternal treatment with short-chain fatty acids modulates the intestinal microbiota and immunity and ameliorates type 1 diabetes in the offspring. PLoS ONE 12, e0183786 (2017).
Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323 (2009).
Gomez de Aguero, M. et al. The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302 (2016).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Singh, R. K. et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 15, 73 (2017).
Srugo, S. A., Bloise, E., Nguyen, T. T. N. & Connor, K. L. Impact of maternal malnutrition on gut barrier defense: implications for pregnancy health and fetal development. Nutrients 11, https://doi.org/10.3390/nu11061375 (2019).
Keleher, M. R. et al. Maternal high-fat diet associated with altered gene expression, DNA methylation, and obesity risk in mouse offspring. PLoS ONE 13, e0192606 (2018).
Thorburn, A. N. et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 6, 7320 (2015).
Hansen, C. H. et al. A maternal gluten-free diet reduces inflammation and diabetes incidence in the offspring of NOD mice. Diabetes 63, 2821–2832 (2014).
Perez-Munoz, M. E., Arrieta, M. C., Ramer-Tait, A. E. & Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5, 48 (2017).
de Goffau, M. C. et al. Human placenta has no microbiome but can contain potential pathogens. Nature 572, 329–334 (2019).
Aagaard, K. et al. The placenta harbors a unique microbiome. Sci. Transl. Med. 6, 237ra265 (2014).
Theis, K. R. et al. No consistent evidence for microbiota in murine placental and fetal tissues. mSphere 5, https://doi.org/10.1128/mSphere.00933-19 (2020).
Stinson, L. F., Boyce, M. C., Payne, M. S. & Keelan, J. A. The not-so-sterile womb: evidence that the human fetus is exposed to bacteria prior to birth. Front. Microbiol. 10, 1124 (2019).
Rackaityte, E. et al. Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med. 26, 599–607 (2020).
Rautava, S., Collado, M. C., Salminen, S. & Isolauri, E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology 102, 178–184 (2012).
Drozdowski, L. & Thomson, A. B. Intestinal hormones and growth factors: effects on the small intestine. World J. Gastroenterol. 15, 385–406 (2009).
Dasgupta, S. & Jain, S. K. Protective effects of amniotic fluid in the setting of necrotizing enterocolitis. Pediatr. Res. 82, 584–595 (2017).
Vidya, M. K. et al. Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int. Rev. Immunol. 37, 20–36 (2018).
Hug, H., Mohajeri, M. H. & La Fata, G. Toll-like receptors: regulators of the immune response in the human gut. Nutrients 10, https://doi.org/10.3390/nu10020203 (2018).
Shibata, T. et al. Toll-like receptors as a target of food-derived anti-inflammatory compounds. J. Biol. Chem. 289, 32757–32772 (2014).
Palmas, F. et al. The choice of amniotic fluid in metabolomics for the monitoring of fetus health. Expert Rev. Mol. Diagn. 16, 473–486 (2016).
Schwarzer, M. et al. Diet matters: endotoxin in the diet impacts the level of allergic sensitization in germ-free mice. PLoS ONE 12, e0167786 (2017).
Nakajima, A. et al. Impact of maternal dietary gut microbial metabolites on an offspring’s systemic immune response in mouse models. Biosci. Microbiota Food Health 39, 33–38 (2020).
American Society for Reproductive, Medicine, American College of Obstetricians and Gynecologists' Committee on Gynecologic Practice. Prepregnancy counseling: Committee Opinion No. 762. Fertil. Steril. 111, 32–42 (2019).
Mousa, A., Naqash, A. & Lim, S. Macronutrient and micronutrient intake during pregnancy: an overview of recent evidence. Nutrients 11, https://doi.org/10.3390/nu11020443 (2019).
Wesolowski, S. R. et al. Switching obese mothers to a healthy diet improves fetal hypoxemia, hepatic metabolites, and lipotoxicity in non-human primates. Mol. Metab. 18, 25–41 (2018).
Acknowledgements
This work was supported by NIH NIDDK R01 DK121975 01A1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Patient consent
No patient consent was required for this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mirpuri, J. Evidence for maternal diet-mediated effects on the offspring microbiome and immunity: implications for public health initiatives. Pediatr Res 89, 301–306 (2021). https://doi.org/10.1038/s41390-020-01121-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01121-x
This article is cited by
-
Butyrate supplementation to pregnant mice elicits cytoprotection against colonic injury in the offspring
Pediatric Research (2022)