Abstract
This manuscript includes (1) a narrative review of Zinc as an essential nutrient for fetal and neonatal growth and brain growth and development and (2) a scoping review of studies assessing the effects of Zinc supplementation on survival, growth, brain growth, and neurodevelopment in neonates. Very preterm infants and small for gestational age infants are at risk for Zinc deficiency. Zinc deficiency can cause several complications including periorificial lesions, delayed wound healing, hair loss, diarrhea, immune deficiency, growth failure with stunting, and brain atrophy and dysfunction. Zinc is considered essential for oligodendrogenesis, neurogenesis, neuronal differentiation, white matter growth, and multiple biological and physiological roles in neurobiology. Data support the possibility that the critical period of Zinc delivery for brain growth in the mouse starts at 18 days of a 20–21-day pregnancy and extends during lactation and in human may start at 26 weeks of gestation and extend until at least 44 weeks of postmenstrual age. Studies are needed to better elucidate Zinc requirement in extremely low gestational age neonates to minimize morbidity, optimize growth, and brain growth, prevent periventricular leukomalacia and optimize neurodevelopment.
Impact
-
Zinc is essential for growth and brain growth and development.
-
In the USA, very preterm small for gestational age infants are at risk for Zinc deficiency.
-
Data support the possibility that the critical period of Zinc delivery for brain growth in the mouse starts at 18 days of a 20–21-day pregnancy and extends during lactation and in human may start at 26 weeks’ gestation and extend until at least 44 weeks of postmenstrual age.
-
Several randomized trials of Zinc supplementation in neonates have shown improvement in growth when using high enough dose, for long duration in patients likely to or proven to have a Zinc deficiency.
-
Studies are needed to better elucidate Zinc requirement in extremely low gestational age neonates to minimize morbidity, optimize growth and brain growth, prevent periventricular leukomalacia and optimize neurodevelopment.
Similar content being viewed by others
Introduction
Insufficient growth in preterm infants, diagnosed by excessive postnatal decreases in Z-scores of weight, length, and fronto-occipital circumference (FOC), but not by percentiles at 36 weeks postmenstrual age (PMA) or discharge, is associated with neurodevelopmental impairment.1 Nutritional factors for brain development include appropriate delivery and uptake of energy, protein, fat, carbohydrate, iron, copper (Cu), zinc (Zn), iodine, thiamine, folate, selenium, choline, vitamins A, B12, C, D, and optimal proportions of long-chain polyunsaturated fatty acids.2,3 Several studies have shown that the developing brain has critical growth periods; however, the critical period for Zn delivery for brain growth has not been established.4,5,6
This manuscript includes (A) a narrative review of the role of Zn as an essential nutrient for fetal and neonatal growth and brain growth and development and (B) a scoping review of the effects of Zn supplementation on survival, growth, brain growth, and neurodevelopment in neonates.
Narrative review
Zinc as an essential nutrient
Role and distribution of Zn
Zn is an essential nutrient. Zn deficiency is an important cause of morbidity and stunting (short length for age and sex) in developing countries worldwide.7,8,9 Zn is one of the most important trace elements in the body as ~10% of the proteins in the human proteome are Zn-dependent. Zn is a component of transcription factors, structural proteins, and enzymes including metalloproteases, nitric oxide synthase, and superoxide dismutase.10,11,12 Most Zn in the body is bound to metallothioneins (MT), a class of proteins important for metal chelation, antioxidant protection, cellular repair processes, nutritional immunity, growth, and differentiation.13 In adults, ~60% of Zn is stored in skeletal muscle, 30% in bone, 5% in liver, and skin.14 Zn is absorbed in the duodenum and jejunum and distributed to all organs, tissues, fluids, and secretions.10 The two families of Zn transporters, Zrt- and Irt-like protein (ZIP) transporters (which increase Zn uptake into the cytoplasm) and ZnT transporters (which reduce cytoplasmic Zn by exporting cellular Zn or by moving it into intracellular organelles or extracellular space) are ubiquitous.14
Assessing body Zn content
Assessing total body Zn content is challenging because Zn is primarily intracellular.15 The Biomarkers of Nutrition for Development (BOND) Zn Expert Panel recommends three measurements for estimating Zn status: dietary Zn intake, plasma Zn concentration, and height-for-age of growing infants and children.15 The amount of dietary Zn intake is higher in diets rich in meat and lower in strict vegetarian or vegan diets and in the breast milk of women with SLC30A2/ZnT2 (ZnT transporter) mutation.10,11 Factors affecting enteral Zn absorption include gastrointestinal diseases, products interfering with Zn absorption (uncooked cereals, geophagy, Cu, iron, and calcium), and mutations of ZIP-4 (Zn transporter protein causing acrodermatitis enteropathica).10,11 A meta-analysis in infants showed a significant relationship between the population mean Zn serum or plasma concentration and Zn intake.16
Most Zn (80%) in the blood is in red blood cells (RBCs) and 87% of RBC Zn is in carbonic anhydrase.17,18,19 In serum, most Zn is bound to albumin and alpha-2 macroglobulin, and a smaller amount is bound to amino acids.20 Serum Zn concentration may decrease with hypoalbuminemia, systemic steroids, infection, acute stress, increased nutritional intake, and growth rate and may be elevated with hemolysis or catabolic state.20,21 Thus, serum Zn concentration is not a gold standard to assess total body Zn content. Potential and emerging biomarkers include hair Zn, urinary Zn, nail Zn, neurobehavioral function, Zn-dependent proteins, oxidative stress, inflammation, and DNA integrity, Zn kinetics, and taste acuity.8,22,23 Zn depletion may occur with only a minimal decrease in hair Zn concentration.23
Role of Zn in growth (Fig. 1)
Zn deficiency limits linear growth, weight gain, and lean body mass accretion. This may be in part related to a reduction in circulating insulin-like growth factor 1 (IGF-1) concentration.11 In a randomized control trial (RCT) in stunted children <2 years of age, Zn supplementation yielded catch-up growth and increased serum concentration of IGF-1.24 However, in neonatal RCTs, Zn supplementation may increase growth without increasing IGF-1 and there is no direct correlation between Zn-related growth response and serum IGF-1 concentration.25,26,27 Zn is required for phosphorylation of the IGF-1 receptor, which is essential for the transduction of the effects of IGF-1. Zn is also required for the activity of deoxythymidine kinase, which converts deoxythymidine into deoxythymidine 5′-monophosphate, a precursor of deoxythymidine triphosphate, which is needed for DNA, protein, and collagen synthesis in rats.11
Zn is the second most abundant metal in the body. Approximately 10% of the proteins in the human proteome are Zn-dependent. Zn is primarily intracellular, where it is stored in MT and is a component of multiple proteins. In the fetus, Zn is stored in liver MT-1, from which it can be released over the first months of postnatal life. Zn interacts with the gut microbiome, immunity, and inflammation. In the brain, Zn has multiple roles in growth, differentiation, and repair. Zn deficiency may result from1 insufficient storage in pregnancy due to severe maternal Zn deficiency, smoking, extreme prematurity, and small size for age;2 insufficient Zn intake due to low Zn concentration in breast milk due to SLC30A2/ZnT2 mutation or maternal Zn deficiency,3 or decreased Zn absorption in the duodenum and jejunum due to SLC39A4/ZIP-4 mutation leading to acrodermatitis enteropathica, or to bowel resection. Zn deficiency may affect multiple transcription factors, storage and structural proteins, enzymes including deoxythymidine kinase, and may decrease the serum concentration of IGF−1 and phosphorylation of the IGF−1 receptor. CA carbonic anhydrase, IGF insulin-like growth factor, MT metallothionein, NMDA N-Methyl-d-aspartate, SLC30A2/ZnT2 mutation leading to lack of Zinc in breast milk, SLC39A4/ZIP-4 mutation leading to acrodermatitis enteropathica, ZIC Zinc finger proteins of the cerebellum, ZIP Zrt- and Irt-like protein, Zn Zinc, ZnT zinc transporter protein.
Zn in pregnancy and fetus
Maternal status in pregnancy
Maternal serum Zn concentration normally decreases until 35 weeks’ gestational age (GA), due to hemodilution, hormonal changes, increased urinary Zn excretion, increased Zn uptake by maternal tissues, and active maternal-fetal transfer of Zn.28 In contrast, RBC Zn concentration increases during pregnancy in parallel with carbonic anhydrase.28
Maternal Zn deficiency
Women with Zn deficiency have lower serum Zn concentration compared to those without deficiency.29 Risk factors for maternal Zn deficiency include digestive disease, bariatric surgery, sickle cell disease, chronic renal disease, smoking, alcoholism, and a vegetarian diet rich in cereals and phytate.30,31,32 Zn deficiency in pregnancy may increase the risk for fetal malformations (e.g., neural tube defects), intrauterine growth restriction (IUGR), and fetal programming of cardiovascular and renal diseases in adult life.33,34,35 However, meta-analyses of RCTs have shown that, while Zn supplementation in pregnancy reduced by 14% the risk of prematurity, it did not improve fetal growth.36,37,38,39,40 The latter finding likely results from multi-nutrient deficiencies.37,39,40
Transplacental transport
Zn is transferred from the mother to the fetus by 2 mechanisms: endocytosis and saturable carrier-facilitated transport.41,42 Zn is taken up against gradient from maternal blood into microvillous borders of human syncytiotrophoblast resulting in Zn storage in the placenta (reaching a concentration of 44 mcg g−1 tissue, ~60× that in plasma), followed by slow passive transfer either bidirectionally or preferentially towards fetal umbilical venous (UV) cord blood.43,44,45,46,47 Adaptation of Zn placental uptake was shown in an in vitro study of microvillous membrane vesicles from preterm and term placentas of Brazilian women.48 Zn uptake was higher in preterm (20–25 weeks GA) than term (>37 weeks) vesicles. In term vesicles, Zn uptake was higher in those from women in the lowest quartile of serum Zn concentration than in those from the highest quartile.48 Placental Zn transport is upregulated in mice with a Zn-deficient diet, as shown by the fact that whole-body Zn fetal uptake in mice is similar to whether the diet in pregnancy is Zn-deficient or Zn-sufficient.49 ZIP and ZnP transporters and MT are expressed in the placenta in mouse, rat, and human.50,51 A RCT suggested the upregulation of ZIP-4 and ZIP-8 mRNAs in the placenta of Gambian women with unsupplemented vs. supplemented Zn.52 However, this study was limited by a lack of assessment of maternal or cord Zn concentration and of ZIP protein expression.52 Smoking in pregnancy results in the upregulation of MT expression in the placenta, which accumulates cadmium instead of Zn.53 In summary, the placenta expresses Zn transporters, takes up Zn from maternal blood, and transports Zn towards the fetus; these processes appear to be upregulated in pregnancies with Zn deficiency.
Serum Zn concentration in the umbilical cord
No study has compared serum Zn concentration in maternal arterial blood and uterine vein with UV and umbilical arterial (UA) blood, which would be the comparisons of choice for analyzing uptake and release in the feto-placental unit.54 All studies comparing maternal to UA and UV Zn concentrations have used peripheral venous maternal blood instead of arterial blood.34 Many studies have shown that serum Zn UV concentration is higher than UA concentration.55,56 However, results are inconsistent across studies. Serum Zn concentration in UA and UV blood or both may be affected by labor, preeclampsia, IUGR, and maternal diabetes and obesity;55,56,57,58,59,60 no data are available in extremely low GA neonates (ELGANs). Cord blood Zn concentration is negatively correlated with GA in studies with many ELGANs, especially AGA infants.61,62 Meta-analysis showed that cord blood serum Zn concentration is lower in small for GA (SGA) or IUGR neonates.35 In summary, cord serum Zn concentration decreases with GA and is lower in SGA and IUGR than in AGA neonates.
Zn accretion in the fetus
Most of Zn accretion in utero occurs during the last trimester of pregnancy; therefore, preterm infants, especially ELGANs, are at risk for Zn deficiency. Accretion of Zn by the human fetus during the third trimester is believed to range between 211 and 270 mcg kg−1 d−1.63 The fetus accumulates Zn in the liver (mostly in MT) at very high concentration, which peaks at 200–1020 mcg g−1 of wet tissue (in US, Japan, and New Zealand) at 22–30 wks GA and later decreases to 140–380 mcg g−1 at term and 50–60 mcg g−1 in infants, children, and adults.64,65,66,67,68 Liver expression of MT decreases during the third trimester of pregnancy and the first months postnatal.64 In the baboon fetus, MT expression decreases progressively in response to increasing maternal estrogen concentration.69 It appears that MT released from the liver may provide an endogenous source of Zn in early postnatal life, possibly up to 2 months postnatal in preterm infants.64,68 In Brazil, where Zn deficiency is prevalent, lower ranges of liver Zn concentration have been reported in autopsies (30–304 mcg g−1 at 26–38 weeks’ gestation, 13–268 mcg g−1 at 40–41 weeks’ gestation, and 3–299 mcg g−1 at <16 weeks post-delivery).70 Smoking mothers have fetuses with lower liver MT expression, which could be due to competition of cadmium with Zn.71 In summary, most Zn accretion by the fetus takes place in the third trimester. Liver Zn concentration peaks at 22–30 weeks and presumably provides an endogenous source of Zn.
Zn in the neonatal period
Zn as an essential nutrient in neonates
There is no consensus about Zn requirements in neonates.12 Using a factorial method taking into account, endogenous liver Zn supply, Klein estimated Zn requirements in preterm infants as 1.5–2 mg kg−1 d−1 for those <1 kg, 1.2–1.7 mg kg−1 d−1 at 1–2 kg, and 1.0–1.3 mg kg−1 d−1 at 2–3 kg.65 Griffin reviewed 11 studies on Zn retention and showed that Zn retention was significantly higher at higher Zn intakes, and higher in formula-based diets than in human milk-based diets.72 Zn intakes of 1.8–2.4 mg kg−1 d−1 (from formula-based diets) and 2.3–2.4 mg kg−1 d−1 (from human milk-based diets) were required to achieve adequate Zn retention to maintain normal growth in preterm infants.72 Intestinal absorption of Zn in preterm infants follows saturable kinetics similar to the adult.73 Zn concentration in breast milk decreases over the early months postpartum, therefore unsupplemented pooled donor breast milk in the USA is not expected to meet Zn requirements for preterm infants.74,75,76 Total parenteral Zn requirement in preterm infants is estimated as 450 mcg kg−1 d−1;77 however, this may be insufficient, especially after bowel resection and enterostomy.
Zn deficiency in neonates
In countries where Zn deficiency is endemic, Zn deficiency at birth is more frequent in SGA especially preterm neonates.78 In the US, Zn deficiency is more likely in SGA ELGANs.79 Zn deficiency has been reported in some breastfed neonates because of very low Zn concentration in breast milk, due to deficiency in ZnT2 transporter.10,80,81 In one study the frequency of significant ZnT2 polymorphisms was 8 among 750 or 1%.82 Low Zn concentration in breast milk can also be secondary to maternal Zn deficiency. In one case, Zn deficiency was reported in a baby born to a mother with Zn deficiency with low breast milk Zn concentration. The baby improved with enteral Zn supplementation and maternal serum and breast milk concentrations improved with enteral Zn supplementation.83
Postnatal Zn deficiency has multiple potential complications including periorificial lesions, delayed wound healing, hair loss, diarrhea, immune deficiency, growth failure with stunting, decreased head growth, and cognitive impairment, which may improve with Zn supplementation.7,11,26,80,81,82,83,84,85,86,87,88 Zn deficiency may contribute to abnormal gut-brain signaling by altering gut physiology and microbiota composition and by triggering an increase of inflammatory markers.89
Assessing Zn deficiency in the neonate
Normal serum Zn concentration in preterm infants decreases during the first weeks postnatal.12 The European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) recommends a normative Zn concentration of 0.74–1.46 mcg ml−1.77 Hair Zn concentrations in term neonates are correlated with maternal hair Zn concentrations.90 In one cohort study in 29 wks preterm infants, hair Zn concentrations decreased by ~40% within 6 months postnatal compared with term infants.90 More data are needed for hair Zn concentrations.15
Zn role in brain growth, differentiation, and repair after injury
Zn role in brain growth and differentiation
Zn affects neuronal differentiation and white matter growth.91 C2H2-type Zn finger proteins are transcription factors that contribute to the regulation of brain morphogenesis, influencing the proliferation, migration, and cell fate of stem cells and neural progenitor cells and their differentiation into neuronal cells.92 The concentration of free Zn in oligodendrocytes decreases from the preoligodendrocyte stage to the mature oligodendrocyte.93 This decrease in Zn concentration may mediate differentiation by modulating transcription factors, enzyme activities, and signaling pathways. mRNA expression of ZnP and ZIP transporters in oligodendrocytes is developmentally regulated in the mouse.94 Expression of ZnT1 protein was shown in oligodendrocytes.95 Saturable Zn uptake was demonstrated in oligodendrocyte progenitors.96 Among those, an oligodendrocyte-specific Zn finger protein (Zfp 488) functions as a transcriptional co-regulator important for oligodendrocyte differentiation.97
In rats, gestational Zn deficiency may cause neural tube defects and other brain malformations and affect brain development (e.g., stem cell proliferation and neuronal number, neuronal specification, myelination, gene expression, N-methyl-d-aspartate [NMDA] receptor expression) and impairs learning and memory into adulthood.91,98,99,100,101 In a model of differentiation of human pluripotent stem cells into motor neurons, mRNA expression of ZnTs and Shank proteins (multidomain scaffold proteins expressed in synapses) was highly regulated during neuronal differentiation.102 In that model, low Zn concentration in the media was associated with increased apoptosis and decreased cell survival, altered neuronal differentiation, and, in particular, synaptic function.102 In summary, Zn may regulate brain ontogeny, neuronal and oligodendrocyte proliferation, differentiation, and function.
In patients with acrodermatitis enteropathica, homozygous or compound loss-of-function mutations in the SLC39A4/ZIP-4 gene result in Zn depletion by blocking gut absorption of Zn; in one case report diffuse cortical atrophy seen on computerized tomography resolved following Zn repletion.103
Critical period of Zn intake for brain growth and differentiation
The critical period of Zn delivery for human brain growth has not been defined.4,6 In the rat, distribution of MT I and II is limited to the septum and hippocampus at birth and progressively involves all forebrain postnatally; vesicular Zn has a similar pattern of development.104 In mice, Zn deprivation starting at 18 days of pregnancy and continuing during lactation reduces weights of pup body, whole brain, and cerebellum during the suckling period when compared with pups from dams fed a diet adequate in Zn.105 In contrast, Zn restriction only in pregnancy or only during the lactational period resulted in smaller changes.105,106 In summary, data in the mouse provides evidence showing that the critical period of Zn delivery for brain growth starts at 18 days of pregnancy and extends during lactation.
In the human brain, MT I and II containing glial cells appear in the subventricular and periventricular zones at 21 weeks of GA and migrate progressively, reaching the entire white and gray matter by 35 weeks GA.107,108 A minor population of late oligodendrocyte progenitors is present in the white matter at 18–27 weeks’ GA, i.e., months before these cells commit to myelinogenesis.109 Starting at 28 weeks GA, the number of immature oligodendrocytes increases, followed by an increase in myelin-binding protein (MBP) and myelin sheets in the periventricular area; this process coincides with the developmental window of vulnerability for periventricular white matter injury.109,110 Limited data suggest that Zn concentration in the human brain progressively decreases from 8 mcg g−1 (wet tissue) at 12 weeks GA to 3–5 mcg g−1 at 23–26 weeks and then increases again to 9 (range 17–22) mcg g−1 at term and in early infancy.67,111,112,113 These data suggest that the critical period of Zn delivery for brain growth is species-specific, i.e., starts at 18 days of pregnancy and extends during lactation in the mouse and could start at 26 weeks and extend until at least 35 weeks of PMA in the human.
Zn role in cerebellar development
Zinc finger proteins of the cerebellum (Zic) may mediate cerebellar developmental control via regulation of neuronal progenitor proliferation-differentiation and the patterning of the cerebellar primordium.114 Zic proteins interact with sonic hedgehog signaling, retinoic acid signaling, and TGFβ signaling during mouse cerebellar development.115 Heterozygous deficiency in Zic1 and Zic4 is associated with Dandy–Walker malformation.115
Zn role in brain injury, degeneration, and repair
Zn has roles in DNA repair, protection against oxidation injury, and repair after ischemia.116,117 Experimental models of ischemic and excitotoxic death acutely alter Zn distribution and increase free Zn concentrations in brain tissue.118,119,120 A block in oligodendrocyte differentiation into MBP-expressing cells is a central problem in periventricular leukomalacia (PVL).121,122 In the chronic stage of PVL cells expressing myelin transcription factor 1, a Zn-dependent DNA binding protein, are significantly increased around necrotic foci and some of the regions are coincident with increasing MBP immunoreactivity.123
Vela et al.124 have reviewed the literature suggesting a possible link between bowel and brain development in autism spectrum disorders. In summary, Zn repletion is important in repair in the chronic phase after brain injury, but not during the acute phase because of increased free Zn concentration in the brain immediately after an ischemic insult.
Scoping review: zinc supplementation for neonatal survival, growth, brain growth, and neurodevelopment
Method
On August 28, 2020, we conducted a PubMed search using the following search words: Zinc supplementation neonate. The Pubmed search engine translated these words into the following strings: (“zinc”[MeSH Terms] OR “zinc”[All Fields]) AND (“supplemental”[All Fields] OR “supplementating”[All Fields] OR “supplementation”[All Fields] OR “supplementation s”[All Fields] OR “supplementations”[All Fields] OR “supplemention”[All Fields]) AND (“infant, newborn”[MeSH Terms] OR (“infant”[All Fields] AND “newborn”[All Fields]) OR “newborn infant”[All Fields] OR “neonatal”[All Fields] OR “neonate”[All Fields] OR “neonates”[All Fields] OR “neonatality”[All Fields] OR “neonatals”[All Fields] OR “neonate s”[All Fields]).
We selected neonatal studies assessing one or more of four outcomes: survival, growth, brain growth, and neurodevelopment. We excluded case reports and studies if they assessed the effects of antenatal Zn supplementation, initiation of Zn supplementation at >28 days (4 weeks) after due dates, and/or supplementation of other nutrients (except Cu to compensate for Zn and Cu competition for gastrointestinal absorption, or studies with factorial design).
Results
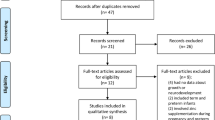
The search yielded 411 manuscripts, among which 33 were assessed on full-text copy and 22 studies met criteria (Fig. 2).
Observational studies
Four studies met the criteria (Table 1).79,86,125,126 In one retrospective study of 60 preterm infants born at 35 ± 1 weeks GA in Egypt, those who received Zn since the first day of life had higher weight and length at the age of 6 months compared with unsupplemented controls.125 The three studies done in the US included FOC measurements. In one retrospective cohort study of 52 ELBW infants (mean GA 25 ± 2 weeks) with chronic lung disease, weight gain increased by 83% and linear growth velocity increased by 57% after supplementing Zn starting at 33 ± 2 weeks PMA for a range of 9.3–43 weeks.126 In a prospective cohort of 105 infants born at 26–37 weeks GA there was a direct relationship between Zn intake and FOC growth.86
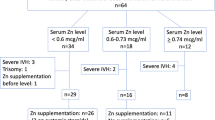
In a prospective cohort study of 302 ELGANs who received recommended Zn intake, a serum Zn concentration was obtained in 52 who had insufficient linear growth; Zn deficiency (serum concentration <0.74 mcg/ml) was diagnosed in 43 infants.79 The odds of Zn deficiency increased in SGA infants and with decreasing GA. In a model including postnatal variables, the odds of Zn deficiency increased with decreasing GA, severe bronchopulmonary dysplasia (BPD), and longer duration of parenteral nutrition.79 In the absence of Zn supplementation, the change in FOC Z-score from time of Zn concentration to discharge or 50 weeks PMA was lower in infants with Zn concentration <0.74 mcg ml−1 than in those with Zn concentration >0.74 mcg ml−1. In Zn-deficient infants, Zn supplementation started at 36 weeks PMA (range 32–44) for >2 weeks but not <2 weeks increased FOC growth rate, but not weight or length growth, in the absence of systemic steroids. The change in FOC Z-score in response to Zn supplementation increased1 with a duration of Zn supplementation (>2 weeks vs. ≤2 weeks),2 lower change in FOC Z-score in response to prior supplementation of protein and3 severe co-morbidity (defined as either severe BPD, gastrointestinal perforation, necrotizing enterocolitis, or severe organ failure). The FOC response to Zn was not affected by PMA at the initiation of therapy. This latter data suggest that the critical period of Zn delivery for brain growth could extend longer than suggested by basic sciences data, i.e., until at least 44 weeks PMA.
Randomized controlled trials
Eighteen studies met the criteria (Table 2).25,26,27,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141 Most studies had some risk of bias limiting the level of evidence (right column, Table 2) and seven studies had no documented sample size analysis. Inclusion criteria were1 SGA, LBW, or IUGR,2 prematurity,3 clinical sepsis,4 or low socioeconomic status. There was substantial heterogeneity in country, duration (10 days–1 year), and a dose of Zn supplementation, type, and timing of assessment tools, and documented or possible confounding variables (micronutrient deficiencies, severe comorbidities, systemic steroids). This heterogeneity limited the validity of a meta-analysis.
Among LBW or preterm neonates in countries where Zn deficiency is prevalent or has been reported in association with stunting (India, Iran, Egypt), prolonged Zn supplementation improved weight in 7/9 studies, length in 6/9, and FOC in 3/5; in other countries (Spain, Italy, USA), respective numbers were 1/4, 2/3, and 0/3.
Five studies assessed the effect of Zn supplementation for at least 8 weeks in SGA or IUGR term infants (studies 1–5, Table 2). Among two studies using a low dose of Zn (1 mg day−1), one showed no effect on growth and no effect on Bayley scores at 6–12 months; the other one, conducted in India, showed that Zn supplementation reduced diarrhea and mortality. Among three studies using higher dose Zn (3–5 mg day−1), 1/3 showed increased weight gain and linear growth.
Among 8 studies enrolling LBW or VLBW infants that were mostly or exclusively preterm (studies 7–14, Table 2), prolonged Zn supplementation improved weight gain in 4/8 studies, linear growth in 5/7, FOC in 3/7, and 2/2 studies showed improved neurologic assessment (one in the NICU and one at 12 months). One Italian RCT using high dose Zn supplementation in VLBW infants showed decreased mortality and composite morbidity (composite of late-onset sepsis, periventricular leukomalacia, necrotizing enterocolitis, retinopathy of prematurity) and increased weight at discharge.84
Three studies assessed short (10 days or until discharge) Zn supplementation (1 or 6 mg kg−1 day−1) for neonates with clinical sepsis (studies 15–17, Table 2). Two of three studies showed no improvement in mortality and one showed no improvement in developmental assessment at 1 year of age. None of these studies assessed growth.
The single study assessing Zn supplementation for low economic status in Chile (study 18, Table 2) showed no effect on growth and showed improvement in Bayley score at 6 months but not at 1 year.
In summary, Zn supplementation was most likely to increase growth in preterm infants in countries where Zn deficiency is prevalent. Zn supplementation may reduce mortality in selected populations. None of the studies assessed important long-term (at least 18 months postnatal age corrected for prematurity) neurodevelopmental outcomes.
Summary
Available data in the mouse provide evidence showing that the critical period of Zn delivery for brain growth starts at 18 days of pregnancy and extends during lactation. Based on limited available data in the human we speculate that the critical period could be from 26 weeks GA until at least 44 weeks PMA.
In countries where Zn deficiency is prevalent, Zn supplementation may help growth in preterm infants and survival in LBW infants. In the US, Zn deficiency is associated with ELGAN, SGA, poor postnatal growth, severe BPD, and prolonged need for parenteral nutrition. Much less frequently, Zn deficiency results from genetic mutations or from maternal Zn deficiency.
Zn deficiency in neonates is best identified with a serum concentration <0.74 mcg/ml and should be treated with Zn supplementation for >2 weeks. Variability in growth response to Zn supplementation depends on several factors:1 patient selection and accuracy of serum level in the detection of Zn deficiency;2 sufficiency of dose and duration of supplementation;3 comorbidities (nutritional, systemic, and medications).
Research gaps
Data are needed to assess markers of total body and brain Zn content in neonates. More data are needed to assess the validity of serum and hair Zn concentration and other biomarkers for this purpose.
No RCT of Zn supplementation was focused on ELGANs or ELBW infants starting before 29 weeks PMA and no RCT has assessed the effect of Zn supplementation on long-term neurodevelopment. Studies are urgently needed to determine the optimal dose, timing, and duration of supplemental Zn in ELGANs that will minimize morbidity, optimize growth and brain growth, prevent periventricular leukomalacia, and optimize neurodevelopment. However, Zn supplementation may not result in optimized growth and brain growth if co-existing nutritional deficiencies exist. Since growth failure is ELGANs is often multifactorial, precision medicine approach may be the best method, requiring a systems biology approach that could include metallomics (assessing trace elements), metabolomics (snapshots of multiple biochemical compounds), and microbiome.89,142,143,144
Change history
02 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41390-021-01425-6
References
Fenton, T. R. et al. “Extrauterine growth restriction” and “postnatal growth failure” are misnomers for preterm infants. J. Perinatol. 40, 704–714 (2020).
Cormack, B. E., Harding, J. E., Miller, S. P. & Bloomfield, F. H. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: a narrative review. Nutrients 11, 2029 (2019).
Valentine, C. J. Nutrition and the developing brain. Pediatr. Res. 87, 190–191 (2020).
Volpe, J. J. Iron and zinc: nutrients with potential for neurorestoration in premature infants with cerebral white matter injury. J. Neonatal Perinat. Med. 12, 365–368 (2019).
Georgieff, M. K., Ramel, S. E. & Cusick, S. E. Nutritional influences on brain development. Acta Paediatr. 107, 1310–1321 (2018).
Georgieff, M. K., Brunette, K. E. & Tran, P. V. Early life nutrition and neural plasticity. Dev. Psychopathol. 27, 411–423 (2015).
Krebs, N. F. Update on zinc deficiency and excess in clinical pediatric practice. Ann. Nutr. Metab. 62(suppl 1), 19–29 (2013).
de Benoist, B., Darnton-Hill, I., Davidsson, L., Fontaine, O. & Hotz, C. Conclusions of the joint WHO/UNICEF/IAEA/IZiNCG interagency meeting on zinc status indicators. Food Nutr. Bull. 28(3 Suppl), S480–S484 (2007).
Wessells, K. R. & Brown, K. H. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 7, e50568 (2012).
Golan, Y., Kambe, T. & Assaraf, Y. G. The role of the zinc transporter SLC30A2/ZnT2 in transient neonatal zinc deficiency. Metallomics 9, 1352 (2017).
Prasad, A. S. Discovery of human zinc deficiency: its impact on human health and disease. Adv. Nutr. 4, 176–190 (2013).
Terrin, G. et al. Zinc in early life: a key element in the fetus and preterm neonate. Nutrients 7, 10427–10446 (2015).
Rahman, M. T. & Karim, M. M. Metallothionein: a potential link in the regulation of zinc in nutritional immunity. Biol. Trace Elem. Res. 182, 1–13 (2018).
Kambe, T., Hashimoto, A. & Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol. Life Sci. 71, 3281–3295 (2014).
King, J. C. et al. Biomarkers of nutrition for development (BOND)-zinc review. J. Nutr. 146, 858S–885S (2015).
Nissensohn, M. et al. Effect of zinc intake on serum/plasma zinc status in infants: a meta-analysis. Matern. Child Nutr. 9, 285–298 (2013). [published correction appears in Matern. Child Nutr. 11, 1056 [2015].
Simons, T. J. Intracellular free zinc and zinc buffering in human red blood cells. J. Membr. Biol. 123, 63–71 (1991).
Ohno, H. et al. A study of zinc distribution in erythrocytes of normal humans. Blut 50, 113–116 (1985).
Moynihan, J. B. Relationship between maturity and isoenzymes of erythrocytic carbonic anhydrase in newborn infants. Pediatr. Res. 11, 871–873 (1977).
Cousins, R. J. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol. Rev. 65, 238–309 (1985).
Terrin, G. Nutritional intake influences zinc levels in preterm newborns: an observational study. Nutrients 19, E529 (2020).
Lowe, N. M. Assessing zinc in humans. Curr. Opin. Clin. Nutr. Metab. Care 19, 321–327 (2016).
Jackson, M. J., Jones, D. A. & Edwards, R. H. Tissue zinc levels as an index of body zinc status. Clin. Physiol. 2, 333–343 (1982).
Cho., J. M., Kim, J. Y. & Yang, H. R. Effects of oral zinc supplementation on zinc status and catch-up growth during the first 2 years of life in children with non-organic failure to thrive born preterm and at term. Pediatr. Neonatol. 60, 201–209 (2019).
Bueno, O. et al. Zinc supplementation in infants with asymmetric intra uterine growth retardation; effect on growth, nutritional status and leptin secretion. Nutr. Hosp. 23, 212–219 (2008).
Díaz-Gómez, N. M. et al. The effect of zinc supplementation on linear growth, body composition, and growth factors in preterm infants. Pediatrics 111, 1002–1009 (2003).
El-Farghali, O., Abd El-Wahed, M., Hassan, N. E., Imam, S. & Alian, K. Early zinc supplementation and enhanced growth of the low-birth weight neonate. Open Access Maced. J. Med. Sci. 3, 63–68 (2015).
Donangelo, C. M. & King, J. C. Maternal zinc intakes and homeostatic adjustments during pregnancy and lactation. Nutrients 4, 782–798 (2012).
Hambidge, K. M. et al. Zinc nutritional status during pregnancy: a longitudinal study. Am. J. Clin. Nutr. 37, 429–442 (1983).
Razagui, I. B. A. & Ghribi, I. Maternal and neonatal scalp hair concentrations of zinc, copper, cadmium, and lead relationship to some lifestyle factors. Biol. Trace Elem. Res. 106, 1–26 (2005).
Kuhnert, P. M. et al. The effect of smoking on placental and fetal zinc status. Am. J. Obstet. Gynecol. 157, 1241–1246 (1987).
Hovdenak, N. & Haram, K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 164, 127–132 (2012).
Moghimi, M., Ashrafzadeh, S., Rassi, S. & Naseh, A. Maternal zinc deficiency and congenital anomalies in newborns. Pediatr. Int. 59, 443–446 (2017).
Tomat, A. L. et al. Morphological and functional effects on cardiac tissue induced by moderate zinc deficiency during prenatal and postnatal life in male and female rats. Am. J. Physiol. Heart Circ. Physiol. 305, H1574–H1583 (2013).
Akdas, S., & Yazihan, N. Cord blood zinc status effects on pregnancy outcomes and its relation with maternal serum zinc levels: a systematic review and meta-analysis. World J. Pediatr. https://doi.org/10.1007/s12519-019-00305-8 (2019).
Ota, E. et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2, CD000230 (2015).
Chaffee, B. W. & King, J. C. Effect of zinc supplementation on pregnancy and infant outcomes: a systematic review. Paediatr. Perinat. Epidemiol. 26(Suppl 1), 118–137 (2012).
Hess, S. Y. & King, J. C. Effects of maternal zinc supplementation on pregnancy and lactation outcomes. Food Nutr. Bull. 30, S60–S78 (2009).
Oh, C., Keats, E. C. & Bhutta, Z. A. Vitamin and mineral supplementation during pregnancy on maternal, birth, child health and development outcomes in low- and middle-income countries: a systematic review and meta-analysis. Nutrients 12, 491 (2020).
Keats, E. C., Haider, B. A., Tam, E. & Bhutta, Z. A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 3, CD004905 (2019).
Bax, C. M. R. & Bloxam, D. L. Two major pathways of zinc(II) acquisition by human placental syncytiotrophoblast. J. Cell. Physiol. 164, 546–554 (1995).
Aslam, N. & McArdle, H. J. Mechanism of zinc uptake by microvilli isolated from human term placenta. J. Cell. Physiol. 151, 533–538 (1993).
Simmer, K., Dwight, J. S., Brown, I. M., Thompson, R. P. & Young, M. Placental handling of zinc in the guinea pig. Biol. Neonate 48, 114–121 (1985).
Beer, W. H. et al. Human placental transfer of zinc: normal characteristics and role of ethanol. Alcohol Clin. Exp. Res. 16, 98–105 (1992).
Nandakumaran, M., Dashti, H. M., Al-Saleh, E. & Al-Zaid, N. S. Transport kinetics of zinc, copper, selenium, and iron in perfused human placental lobule in vitro. Mol. Cell Biochem. 252, 91–96 (2003).
Mas, A. & Sarkar, B. Binding, uptake and efflux of 65Zn by isolated human trophoblast cells. Biochim. Biophys. Acta 1092, 35–38 (1991).
Osada, H. et al. Profile of trace element concentrations in the feto-placental unit in relation to fetal growth. Acta Obstet. Gynecol. Scand. 81, 931–937 (2002).
Zapata, C. L., Melo, M. R. & Donangelo, C. M. Maternal, placental and cord zinc components in healthy women with different levels of serum zinc. Biol. Neonate 72, 84–93 (1997).
Matsusaka, N. et al. Whole-body retention and fetal uptake of 65Zn in pregnant mice fed a Zn-deficient diet. J. Radiat. Res. 36, 196–202 (1995).
Asano, N. et al. Expression profiles of zinc transporters in rodent placental models. Toxicol. Lett. 154, 45–53 (2004).
Ford, D. Intestinal and placental zinc transport pathways. Proc. Nutr. Soc. 63, 21–29 (2004).
Jobarteh, M. L. et al. mRNA levels of placental iron and zinc transporter genes are upregulated in gambian women with low iron and zinc status. J. Nutr. 147, 1401–1409 (2017).
Ronco., A. M., Arguello, G., Suazo, M. & Llanos, M. N. Increased levels of metallothionein in placenta of smokers. Toxicology 208, 133–139 (2005).
Holm, M. B. et al. Uptake and release of amino acids in the fetal-placental unit in human pregnancies. PLoS ONE 12, e0185760 (2017).
Al-Saleh, E., Nandakumaran, M., Al-Shammari, M., Al-Falah, F. & Al-Harouny, A. Assessment of maternal-fetal status of some essential trace elements in pregnant women in late gestation: relationship with birth weight and placental weight. J. Matern. Fetal Neonatal Med. 16, 9–14 (2004).
Katz, O. et al. Severe pre-eclampsia is associated with abnormal trace elements concentrations in maternal and fetal blood. J. Matern. Fetal Neonatal Med. 25, 1127–1130 (2012).
Lazer, T. et al. Trace elements′ concentrations in maternal and umbilical cord plasma at term gestation: a comparison between active labor and elective cesarean delivery. J. Matern. Fetal Neonatal Med. 25, 286–289 (2012).
Al-Saleh, E., Nandakumaran, M., Al-Shammari, M. & Al-Harouny, A. Maternal-fetal status of copper, iron, molybdenum, selenium and zinc in patients with gestational diabetes. J. Matern. Fetal Neonatal Med. 16, 15–21 (2004).
Al-Saleh, E. et al. Maternal-fetal status of copper, iron, molybdenum, selenium and zinc in insulin-dependent diabetic pregnancies. Arch. Gynecol. Obstet. 271, 212–217 (2005).
Krachler, M., Rossipal, E. & Micetic-Turk, D. Trace element transfer from the mother to the newborn investigations on triplets of colostrum, maternal and umbilical cord sera. Eur. J. Clin. Nutr. 53, 486–494 (1999).
Galinier, A. et al. Reference range for micronutrients and nutritional marker proteins in cord blood of neonates appropriated for gestational ages. Early Hum. Dev. 81, 583–593 (2005).
Kojima, C. et al. Association of zinc and copper with clinical parameters in the preterm newborn. Pediatr. Int. 59, 1165–1168 (2017).
Shaw, J. C. Trace elements in the fetus and young infant. I. Zinc. Am. J. Dis. Child. 133, 1260–1268 (1979).
Klein, D., Scholz, P., Drasch, G. A., Müller-Höcker, J. & Summer, K. H. Metallothionein, copper and zinc in fetal and neonatal human liver: changes during development. Toxicol. Lett. 56, 61–67 (1991).
Klein, C. J. Nutrient requirements for preterm infant formulas. J. Nutr. 132(Suppl. 1), 1395S–1577S (2002). 6.
Casey, C. E. & Robinson, M. F. Copper, manganese, zinc, nickel, cadmium and lead in human foetal tissues. Br. J. Nutr. 39, 639–646 (1978).
Chaube, S., Nishimura, H. & Swinyard, C. A. Zinc and cadmium in normal human embryos and fetuses: analyses by atomic absorption spectrophotometry. Arch. Environ. Health 26, 237–240 (1973).
Zlotkin, S. H. & Cherian, M. G. Hepatic metallothionein as a source of zinc and cysteine during the first year of life. Pediatr. Res. 24, 326–329 (1988).
Rosenthal, M. D., Albrecht, E. D. & Pepe, G. J. Estrogen modulates developmentally regulated gene expression in the fetal baboon liver. Endocrine 23, 219–228 (2004).
Dorea, J. G., Brito, M. & Araujo, M. O. Concentration of copper and zinc in liver of fetuses and infants. J. Am. Coll. Nutr. 6, 491–495 (1987).
Bilde, K. et al. Reduced hepatic metallothionein expression in first trimester fetuses in response to intrauterine smoking exposure: a consequence of low maternal zinc levels? Hum. Reprod. 34, 2129–2143 (2019).
Griffin, I. J., Domellöf, M., Bhatia, J., Anderson, D. M. & Kler, N. Zinc and copper requirements in preterm infants: an examination of the current literature. Early Hum. Dev. 89, S29–S34 (2013).
Hambidge, K. M., Krebs, N. F., Westcott, J. E. & Miller, L. V. Changes in zinc absorption during development. J. Pediatr. 149, S64–S68 (2006).
Djurović, D. et al. Zinc concentrations in human milk and infant serum during the first six months of lactation. J. Trace Elem. Med. Biol. 41, 75–78 (2017).
Young, B. E. et al. Effect of pooling practices and time postpartum of milk donations on the energy, macronutrient, and zinc concentrations of resultant donor human milk pools. J. Pediatr. 214, 54–59 (2019).
Casey, C. E., Neville, M. C. & Hambidge, K. M. Studies in human lactation: secretion of zinc, copper, and manganese in human milk. Am. J. Clin. Nutr. 49, 773–785 (1989).
Finch, C. W. Review of trace mineral requirements for preterm infants: what are the current recommendations for clinical practice?. Nutr. Clin. Pr. 30, 44–58 (2015).
Gupta, N., Bansal, S., Gupta, M. & Nadda, A. A comparative study of serum zinc levels in small for gestational age babies and appropriate for gestational age babies in a Tertiary Hospital, Punjab. J. Fam. Med. Prim. Care 9, 933–937 (2020).
Brion, L. P. et al. Zinc deficiency limiting head growth to discharge in extremely low gestational age infants with insufficient linear growth: a cohort study [published online ahead of print, 2020 Aug 12]. J. Perinatol. https://doi.org/10.1038/s41372-020-00778-w (2020)
Kienas, A., Roth, B., Bossier, C., Jojabri., C. & Hoeger, P. H. Zinc-deficiency dermatitis in breast-fed infants. Eur. J. Pediatr. 166, 189–194 (2007).
Itsumura, N. et al. Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: a novel mechanism for zinc deficiency in a breast-fed infant. PLoS ONE 8, e64045 (2013).
Qian, L., Wang, B., Tang, N., Zhang, W. & Cai, W. Polymorphisms of SLC30A2 and selected perinatal factors associated with low milk zinc in Chinese breastfeeding women. Early Hum. Dev. 88, 663–668 (2012).
Vashist, S., Rana, A. & Mahajan, V. K. Transient symptomatic zinc deficiency in a breastfed infant associated with low zinc levels in maternal serum and breast milk improving after zinc supplementation: an uncommon phenotype? Indian Dermatol. Online J. 11, 623–626 (2020).
Terrin, G. et al. Zinc supplementation reduces morbidity and mortality in very-low birth-weight preterm neonates: a hospital-based randomized, placebo-controlled trial in an industrialized country. Am. J. Clin. Nutr. 98, 1468–1474 (2013).
Sjöström, E. S., Öhlund, I., Ahlsson, F. & Domellöf, M. Intakes of micronutrients are associated with early growth in extremely preterm infants. J. Pediatr. Gastroenterol. Nutr. 62, 885–892 (2016).
Harris, T., Gardner, F., Podany, A., Kelleher, S. L. & Doheny, K. K. Increased early enteral zinc intake improves weight gain in hospitalised preterm infants. Acta Paediatr. 108, 1978–1984 (2019).
Haase, H. & Rink, L. Functional significance of zinc-related signaling pathways in immune cells. Annu. Rev. Nutr. 29, 133–152 (2009).
Abdollahi, M., Abdollahi, Z., Fozouni, F. & Bondarianzadeh, D. Oral zinc supplementation positively affects linear growth, but not weight, in children 6-24 months of age. Int. J. Prev. Med. 5, 280–286 (2014).
Sauer, A. K. & Grabrucker, A. M. Zinc deficiency during pregnancy leads to altered microbiome and elevated inflammatory markers in mice. Front. Neurosci. 13, 1295 (2019).
Friel, J. K., Gibson, R. S., Balassa, R. & Watts, J. L. A comparison of the zinc, copper and manganese status of very low birth weight pre-term and full-term infants during the first twelve months. Acta Paediatr. Scand. 73, 596–601 (1984).
Levenson, C. W. & Morris, D. Zinc and neurogenesis: making new neurons from development to adulthood. Adv. Nutr. 2, 96–100 (2011).
Al-Naama, N., Mackeh, R. & Kino, T. C2H2-type zinc finger proteins in brain development, neurodevelopmental, and other neuropsychiatric disorders: systematic literature-based analysis. Front. Neurol. 11, 32 (2020).
Bourassa, D. et al. Chromis-1, a ratiometric fluorescent probe optimized for two-photon microscopy reveals dynamic changes in labile Zn(II) in differentiating oligodendrocytes. A. C. S. Sens. 3, 458–467 (2018).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex [published correction appears in J Neurosci 34, 11929–11947 (2014)]. J. Neurosci. 35, 846 (2015).
Nolte, C. et al. ZnT-1 expression in astroglial cells protects against zinc toxicity and slows the accumulation of intracellular zinc. Glia 48, 145–155 (2004).
Law, W., Kelland, E. E., Sharp, P. & Toms, N. J. Characterisation of zinc uptake into rat cultured cerebrocortical oligodendrocyte progenitor cells. Neurosci. Lett. 352, 113–116 (2003).
Wang, S. Z. et al. An oligodendrocyte-specific zinc-finger transcription regulator cooperates with Olig2 to promote oligodendrocyte differentiation. Development 133, 3389–3398 (2006).
Adamo, A. M. & Oteiza, P. I. Zinc deficiency and neurodevelopment: the case of neuron. Biofactors 36, 117–124 (2010).
Chowanadisai, W., Kelleher, S. L. & Lönnerdal, B. Maternal zinc deficiency reduces NMDA receptor expression in neonatal rat brain, which persists into early adulthood. J. Neurochem. 94, 510–519 (2005).
Liu, H., Oteiza, P. I., Gerswhin, M. E., Golub, M. S. & Keen, C. L. Effects of maternal marginal zinc deficiency on myelin protein profiles in the suckling rat and infant rhesus monkey. Biol. Trace Elem. Res. 34, 55–66 (1992).
Gower-Winter, S. D., Corniola, R. S., Morgan, T. J. Jr. & Levenson, C. W. Zinc deficiency regulates hippocampal gene expression and impairs neuronal differentiation. Nutr. Neurosci. 16, 174–182 (2013).
Pfaender, S. et al. Cellular zinc homeostasis contributes to neuronal differentiation in human induced pluripotent stem cells. Neural Plast. 2016, 3760702 (2016).
Ohlsson, A. Acrodermatitis enteropathica. Reversibility of cerebral atrophy with zinc therapy. Acta Paediatr. 70, 269–273 (1981).
Penkowa, M., Nielsen, H., Hidalgo, J., Bernth, N. & Moos, T. Distribution of metallothionein I + II and vesicular zinc in the developing central nervous system: correlative study in the rat. J. Comp. Neurol. 412, 303–318 (1999).
Prohaska, J. R., Luecke, R. W. & Jasinski, R. Effect of zinc deficiency from day 18 of gestation and/or during lactation on the development of some rat brain enzymes. J. Nutr. 11, 1525–1531 (1974).
Azman, M. S., Wan Saudi, W. S., Ilhami, M., Mutalib, M. S. & Rahman, M. T. Zinc intake during pregnancy increases the proliferation at ventricular zone of the newborn brain. Nutr. Neurosci. 12, 9–12 (2009).
Cozzi, B. et al. Ontogenesis and migration of metallothionein I/II-containing glial cells in the human telencephalon during the second trimester. Brain Res. 1327, 16–23 (2010).
Suzuki, K., Nakajiama, K., Otaki, N. & Kimura, M. Metallothionein in developing human brain. Biol. Signals 3, 188–192 (1994).
Back, S. A., Luo, N. L., Borenstein, N. S., Volpe, J. J. & Kinney, H. C. Arrested oligodendrocyte lineage progression during human cerebral white matter development: Dissociation between the timing of progenitor differentiation and myelinogenesis. J. Neuropathol. Exp. Neurol. 61, 197–211 (2002).
Back, S. A. et al. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 21, 1302–1312 (2001).
Vahter, M. et al. Concentrations of copper, zinc and selenium in brain and kidney of second trimester fetuses and infants. J. Trace Elem. Med. Biol. 11, 215–222 (1997).
Höck, A., Demmel, U., Shicka, H., Kasperek, K. & Feinendegen, L. E. Trace element concentration in human brain. Activation analysis of cobalt, iron, rubidium, selenium, zinc, chromium, silver, cesium, antimonium and scandium. Brain 98, 49–64 (1975).
Gélinas, Y., Lafond, J. & Schmidt, J. P. Multielemental analysis of human fetal tissues using inductively coupled plasma-mass spectrometry. Biol. Trace Elem. Res. 59, 63–74 (1997).
Aruga, J. & Millen, K. J. ZIC1 function in normal cerebellar development and human developmental pathology. Adv. Exp. Med. Biol. 1046, 249–268 (2018).
Grinberg, I. et al. Heterozygous deletion of the linked genes ZIC1 and ZIC4 is involved in Dandy-Walker malformation. Nat. Genet. 36, 1053–1055 (2004).
Frederickson, C. J., Koh, J. Y. & Bush, A. I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 6, 449–462 (2005).
Qi, Z. & Liu, K. J. The interaction of zinc and the blood-brain barrier under physiological and ischemic conditions. Toxicol. Appl. Pharm. 364, 114 (2019).
Zhang, Y., Aizenman, E., DeFranco, D. B. & Rosenberg, P. A. Intra-cellular zinc release, 12-lipoxygenase activation and MAPK dependent neuronal and oligodendroglial death. Mol. Med. 13, 350–355 (2007).
Domercq, M. et al. Zn2+-induced ERK activation mediates PARP-1-dependent ischemic-reoxygenation damage to oligodendrocytes. Glia 61, 383–393 (2013).
Mato, S., Sanchez-Gomez, M. V., Bernal-Chico, A. & Matute, C. Cytosolic zinc accumulation contributes to excitotoxic oligodendroglial death. Glia 61, 750–764 (2013).
Buser, J. R. et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 71, 93–109 (2012).
Segovia, K. N. et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann. Neurol. 63, 520–530 (2008).
Hirayama, A. et al. Myelin transcription factor 1 (MyT1) immunoreactivity in infants with periventricular leukomalacia. Brain Res. Dev. Brain Res. 140, 85–92 (2003).
Vela, G. et al. Zinc in gut-brain interaction in autism and neurological disorders. Neural Plast. 2015, 972791 (2015).
El Mashad, G. M., El Sayed, H. M. & Elghorab, A. M. S. Effect of zinc supplementation on growth of preterm infants. Menoufia Med. J. 29, 1112–1115 (2016).
Shaikhkhalil, A. K., Curtiss, J., Puthoff, T. D. & Valentinem, C. J. Enteral zinc supplementation and growth in extremely-low-birth-weight infants with chronic lung disease. J. Pediatr. Gastroenterol. Nutr. 58, 183–187 (2014).
Castillo-Durán, C., Rodríguez, A., Venegas, G., Alvarez, P. & Icaza, G. Zinc supplementation and growth of infants born small for gestational age. J. Pediatr. 127, 206–211 (1995).
Ashworth, A., Morris, S. S., Lira, P. I. & Grantham-McGregor, S. M. Zinc supplementation, mental development and behaviour in low birth weight term infants in northeast Brazil. Eur. J. Clin. Nutr. 52, 223–227 (1998).
Lira, P. I., Ashworth, A. & Morris, S. S. Effect of zinc supplementation on the morbidity, immune function, and growth of low-birth-weight, full-term infants in northeast Brazil. Am. J. Clin. Nutr. 68(2 Suppl), 418S–424S (1998).
Sazawal, S. et al. Zinc supplementation in infants born small for gestational age reduces mortality: a prospective, randomized, controlled trial. Pediatrics 108, 1280–1286 (2001).
Taneja, S. et al. Effect of zinc supplementation on morbidity and growth in hospital-born, low-birth-weight infants. Am. J. Clin. Nutr. 90, 385–391 (2009).
Sur, D. et al. Impact of zinc supplementation on diarrheal morbidity and growth pattern of low birth weight infants in kolkata, India: a randomized, double-blind, placebo-controlled, community-based study. Pediatrics 112, 1327–1332 (2003).
Friel, J. K. et al. Zinc supplementation in very-low-birth-weight infants. J. Pediatr. Gastroenterol. Nutr. 17, 97–104 (1993).
Islam, M. N. et al. Effect of oral zinc supplementation on the growth of preterm infants. Indian J. Pediatr. 47, 845–849 (2010).
Aminisani, N., Barak, M. & Shamshirgaran, S. M. Effect of zinc supplementation on growth of low birth weight infants aged 1-6 mo in Ardabil, Iran. Indian J. Pediatr. 78, 1239–1243 (2011).
Kumar, T. V. R. & Ramji, S. Effect of zinc supplementation on growth in very low birth weight infants. J. Trop. Pediatr. 58, 50–54 (2012).
Mahtur, N. B. & Agarwal, D. K. Zinc supplementation in preterm neonates and neurological development: a randomized controlled trial. Indian Pediatr. 52, 951–955 (2015).
Mehta, K., Bhatta, N. K., Majhi, S., Shrivastava, M. K. & Singh, R. R. Oral zinc supplementation for reducing mortality in probable neonatal sepsis: a double-blind randomized placebo-controlled trial. Indian Pediatr. 50, 390–393 (2013).
Newton, B., Bhat, B. V., Dhas, B. B., Mondal, N. & Gopalakrishna, S. M. Effect of zinc supplementation on early outcome of neonatal sepsis-a randomized controlled trial. Indian J. Pediatr. 83, 289–293 (2016).
Banupriya, N. et al. Short-term oral zinc supplementation among babies with neonatal sepsis for reducing mortality and improving outcome—a double-blind randomized controlled trial. Indian J. Pediatr. 85, 5–9 (2018).
Castillo-Durán, C. et al. Effect of zinc supplementation on development and growth of Chilean infants. J. Pediatr. 138, 229–235 (2001).
Souza, R. T. et al. Metabolomics applied to maternal and perinatal health: a review of new frontiers with a translation potential. Clinics 74, e894 (2019).
Gracie, S. et al. An integrated systems biology approach to the study of preterm birth using "-omic" technology-a guideline for research. B. M. C. Pregnancy Childbirth 11, 71 (2011).
Liu, J., Chen, X. X., Li, X. W., Fu, W. & Zhang, W. Q. Metabolomic research on newborn infants with intrauterine growth restriction. Medicine 95, e3564 (2016).
Funding
This study was funded by the Children’s Health, Dallas: Senior Investigator Research Award (CCRAC)–New Direction (L.P.B.).
Author information
Authors and Affiliations
Contributions
L.P.B. wrote the first draft of the manuscript, critically reviewed the revisions, and approved the final manuscript as submitted. R.H. and C.S.L. critically reviewed the revisions and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brion, L.P., Heyne, R. & Lair, C.S. Role of zinc in neonatal growth and brain growth: review and scoping review. Pediatr Res 89, 1627–1640 (2021). https://doi.org/10.1038/s41390-020-01181-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01181-z
This article is cited by
-
Associations of Perinatal Metal and Metalloid Exposures with Early Child Behavioral Development Over Time in the New Hampshire Birth Cohort Study
Exposure and Health (2024)
-
Current understanding of essential trace elements in intrahepatic cholestasis of pregnancy
BioMetals (2024)
-
Hyperglycemia and prematurity: a narrative review
Pediatric Research (2023)
-
Growth after implementing a donor breast milk program in neonates <33 weeks gestational age or birthweight <1500 grams: Retrospective cohort study
Journal of Perinatology (2023)
-
Effect of enteral zinc supplementation on growth and neurodevelopment of preterm infants: a systematic review and meta-analysis
Journal of Perinatology (2022)