Abstract
Neonatal sepsis accounts for significant morbidity and mortality, particularly among premature infants in the Neonatal Intensive Care Unit. Abnormal vital sign patterns serve as physiomarkers of sepsis and provide early warning of illness before overt clinical decompensation. The systemic inflammatory response to pathogens signals the autonomic nervous system, leading to changes in temperature, respiratory rate, heart rate, and blood pressure. In infants with comorbidities of prematurity, vital sign abnormalities often occur in the absence of infection, which confounds sepsis diagnosis. This review will cover the mechanisms of vital sign changes in neonatal sepsis, including the cholinergic anti-inflammatory pathway mediated by the vagus nerve, which is critical to the host response to infectious and inflammatory insults. We will also review the clinical implications of vital sign changes in neonatal sepsis, including their use in early warning scores and systems to direct clinicians to the bedside of infants with physiologic changes that might be due to sepsis.
Impact
-
This manuscript summarizes and reviews the relevant literature on the physiological manifestations of neonatal sepsis and how we monitor and analyze these through vital signs and advanced analytics.
Similar content being viewed by others
Introduction: vital sign changes in neonatal sepsis
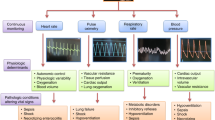
Vital sign changes occur in neonates as part of a systemic inflammatory response to sepsis. Following pathogen invasion into tissues or the bloodstream, immune cells release cytokines, chemokines, and other inflammatory mediators and modulators in a signaling cascade leading to an increasingly robust physiologic syndrome. The response to sepsis, mediated in part by the autonomic nervous system, manifests as changes in body temperature, respiratory drive and oxygenation, heart rate characteristics, and blood pressure. These signs may be subtle in the initial stages of sepsis and severe in the advanced stages. Monitoring continuous vital sign trends or patterns can detect changes in physiology before overt clinical deterioration and can add information when clinical signs are subtle or non-specific.1,2,3
For adults and children, vital sign criteria for the systemic inflammatory response syndrome (SIRS), sepsis, and septic shock include specific thresholds for tachypnea, tachycardia, hypotension, and fever or hypothermia.4 Abnormal vital sign thresholds and SIRS criteria are less useful in neonates, particularly those born preterm, due to difficulties in establishing gestational and chronologic age-based normal ranges.5 Neonates’ vital signs can change in either direction in the setting of sepsis, especially for those born preterm. They may present with hypothermia or fever, apnea or tachypnea, and bradycardia or tachycardia.
The autonomic nervous system (ANS) is the primary link between inflammation and vital sign changes in sepsis. Cytokines, microbial toxins, and immune cells activate the ANS rapidly following pathogen invasion. The sympathetic nervous system (SNS) processes the signal to eliminate a pathogen and launches the “flight or fight” response via catecholamine release and adrenal stimulation, which may be manifest as tachycardia, tachypnea, and changes in blood pressure. The parasympathetic nervous system (PNS) provides a system of checks and balances by way of the cholinergic anti-inflammatory pathway. This response, mediated by the vagus nerve, is essential for survival in preclinical models of sepsis.6,7
Vital sign interactions occur as part of healthy, networked physiology that may be disrupted during sepsis.8,9 For example, respiratory sinus arrhythmia is a phenomenon mediated by the baroreflex that results in a rise and fall in the heart rate with inhalation and exhalation, respectively.10 Measures of cardiorespiratory interaction and autonomic tone increase in premature infants as they mature,11,12 and are generally associated with healthier patients or outcomes.13 Detection of the loss of physiological interactions through continuous analysis of vital sign patterns might provide early warning of impending clinical decompensation.14
This review will discuss abnormalities of specific vital signs in septic neonates, focusing on mechanisms and clinical implications. We will also consider the role of the autonomic nervous system in the systemic inflammatory response to sepsis and how this manifests as vital sign changes. Finally, we will review scores and systems that incorporate vital signs and serve as sepsis early warning systems or predictive analytics for patients in the NICU.
Classifying neonatal sepsis using aberrant vital signs and organ dysfunction
The current definition of neonatal sepsis requires a positive blood culture obtained due to clinical signs of illness, treated with a full course of antibiotics. This definition lacks criteria for illness severity, which limits the analysis of outcomes and responses to interventions given the wide range of clinical presentations.15 For adults, in 2001, the International Sepsis Definitions conference refined the criteria for systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock, including specific criteria for respiratory rate, heart rate, temperature, and blood pressure. Criteria for children and term neonates were proposed in 2002,16 but these consensus statements excluded preterm infants who, for several reasons, have defied threshold-based sepsis definitions.5,17 Even for adults, SIRS criteria no longer define sepsis, with newer guidelines based instead on organ dysfunction using the sequential organ failure assessment (SOFA) score.18
Pathogen and host variability and impact on vital sign changes
The broad range of sepsis severity can be attributed to patient-specific variables such as age,19 sex, genetic factors,20 and comorbidities, as well as pathogen-specific variables such as the ability to invade tissues, replicate, evade the immune system, and damage tissues.21,22 Low gestational and postnatal age correspond to less mature innate and acquired immunity leading to a heightened risk for overwhelming sepsis, shock, and death.23 Technological support provided to NICU patients can also impact changes in vital signs.24,25,26 For example, mechanical ventilation masks apnea, a common presenting sign of sepsis for preterm infants.27 Medications can also impact vital signs and confound their use as physiomarkers of sepsis. For example, caffeine or beta-adrenergic agonist drugs may raise the heart rate, and anticholinergic medications cause tachycardia and decreased heart rate variability. Glucocorticoids augment heart rate variability28 and may modify fever and hypotension.
Sex and race play a role in the immune response to infection29 and may impact changes in vital signs. A recent study showed lower oxygen saturation values in adults with darker skin color compared to those with lighter skin.30 With regard to sex, adult females have been reported to have higher CRP levels,31 higher heart rate, and lower heart rate variability compared to males.32 The impact of sex and race on vital signs and immunity in neonates is not well understood. Host factors affecting infection risk and response also include genetic polymorphisms. Single nucleotide polymorphisms have been identified and characterized for receptors that recognize pathogens, such as toll-like receptors, and in mediators of the inflammatory response, such as cytokines, and in their effect on the risk of infection and the inflammatory response to pathogens.33,34,35,36 For example, a study in premature infants found an association between a TLR4 single nucleotide polymorphism and an increased risk of gram-negative infections.33
Pathogen virulence properties also play a role in the epidemiology of infections and the severity of associated vital sign changes in sepsis. The most common bloodstream pathogen in preterm infants is coagulase-negative staphylococcus (CONS) which, although implicated in severe sepsis,37 generally causes much less severe illness and carries a lower risk of morbidity and mortality than Gram-negative or other Gram-positive bacteria.38 The immune response to CONS appears to be less robust, as evidenced by lower levels of circulating pro-inflammatory cytokines39 and less increase in other acute phase reactants such as C-reactive protein (CRP) or procalcitonin.40 Although some studies suggest that CRP may be useful to differentiate CONS sepsis from blood culture contamination to reduce unnecessary antibiotic exposure,.41 other studies on the diagnostic accuracy of CRP for neonatal sepsis report heterogeneity in measures of sensitivity and specificity,42 likely due to pathogen and host variability
Evidence of increased virulence of Gram-negative bacteria includes an NICHD Neonatal Network reported 36% mortality with Gram-negative sepsis versus 11% with Gram-positive organisms in very low birth weight (VLBW) infants.43 This variability is due in part to differences in pathogen-associated molecular patterns (PAMPs),44 with lipopolysaccharide on Gram-negative bacteria eliciting a greater degree of immune activation than Gram-positive peptidoglycan and lipoteichoic acid.45
Sepsis due to viruses also occurs in NICU patients, although less often, and viral testing is rarely performed as a routine part of a sepsis evaluation.46 An important part of the antiviral response includes induction of interferons, such as IFN-alpha (IFN-α), which can suppress C-reactive protein production, making CRP a poor biomarker of viral infections.47,48 These and other differences in pathogen virulence properties account, in part, for variability in clinical presentation, including vital sign changes.
Abnormal heart rate characteristics in neonatal sepsis: mechanisms
Tachycardia: Pathogen endotoxins, cytokines, and other immune modulators trigger autonomic nervous system activation via afferent signals to the brainstem.49,50 Upon activation of the sympathetic nervous system, preganglionic neurons relay signals to the spinal ganglia via nicotinic receptors which signal postganglionic neurons to release catecholamine at their target organs.50 Norepinephrine binds to adrenergic receptors at the sinus node, and ion channel modifications result in shorter action potential time and a faster heart rate.50 Since the baseline heart rate of neonates is already relatively high at 140–150 beats per minute, any further increases during sepsis may be insufficient to maintain adequate tissue perfusion.
Intravascular volume depletion due to endothelial damage and capillary leak can also lead to tachycardia. Decreased preload leads to a compensatory increase in heart rate to maintain cardiac output. The neonatal heart has decreased elasticity and left ventricular muscle mass, making it less able to increase stroke volume to maintain cardiac output.51,52 Tachycardia in sepsis may prevent adequate diastolic filling, which can, in turn, lead to myocardial ischemia and dysfunction.
Bradycardia: The PNS works rapidly to sense pathogens or inflammation, sends signals to the brainstem to activate anti-inflammatory pathways, and signals acetylcholine release to inhibit cytokine synthesis as a reflexive brake on the inflammatory response.53 Acetylcholine at the sinus node slows the time it takes to generate an action potential and therefore slows the heart rate. Transient, repetitive decelerations in heart rate occur in neonates with sepsis, likely due to vagus nerve firing as part of the cholinergic anti-inflammatory response. In a preclinical model, mice injected with Gram-positive or Gram-negative bacteria or Candida albicans had repetitive heart rate decelerations within seconds of pathogen injection, associated with activation of vagal nuclei in the brainstem, and these decelerations were blocked by anticholinergic medication (atropine).54 In preterm infants, another mechanism of bradycardia is central apnea which often increases in sepsis due to endogenous prostaglandin production.55,56
Depressed Heart rate variability: Abnormally low heart rate variability signifies ANS dysfunction due to acute or chronic pathology across the age spectrum, including and especially apparent in sepsis.57,58 The ANS controls the heart rate by varying impulse traffic in autonomic nerve fibers originating in the brainstem and terminating at the sinoatrial node. Normally, homeostatic signaling creates balanced fluctuations in the sympathetic and parasympathetic nervous systems via norepinephrine and acetylcholine release, resulting in frequent small accelerations and decelerations of heart rate. The rise and fall of intrathoracic pressure during respiration influence this balance through the baroreflex, which contributes to a phenomenon called respiratory sinus arrhythmia (RSA).59,60
Cardiorespiratory coupling may be a surrogate measure of autonomic function, specifically from vagal input. In healthy infants, RSA is most notable during quiet sleep.61 In frequency-domain analysis, the heart rate power spectrum in the high-frequency range correlates with the breathing rate and is generally viewed as a measure of the degree of RSA or vagal tone.62 In adults with a heart rate over twice that of their respiratory rate, this measure and interpretation is feasible and generally sound. In premature infants with a respiratory rate sometimes equal to their heart rate, frequency-domain HRV analysis is limited by the mathematic principles involved.63 Analysis of HRV maturation in both the time series and frequency domain demonstrates an increase in HRV from birth to term corrected age in infants without major morbidities12 as the ANS develops.
In the fetus, decreased HRV is a well-recognized sign of distress, associated with poor placental perfusion, hypoxia, or chorioamnionitis.64,65,66 In neonates, low HRV reflects acute or chronic ANS dysfunction due to brain injury,67,68,69 focal or systemic inflammation without infection,70 or an acute systemic inflammatory response to sepsis or other infections.71,72,73,74 Furthermore, HRV improves during recovery from illness.75 The mechanism of decreased HRV in sepsis is, at least in part, related to cytokine release.76 In a mouse model of sepsis, administration of bacterial lipopolysaccharide led to cytokine production and decreased heart rate variability, both of which were attenuated by glucocorticoid pre-treatment. Administration of pro-inflammatory cytokines alone also depressed heart rate variability.77
Clinical implications of heart rate changes in sepsis
Persistent tachycardia, a decline in heart rate variability, and repetitive, transient bradycardia spells can be early markers of sepsis in NICU patients. Depressed heart rate variability is difficult to discern using conventional NICU monitoring, and pathologic heart rate decelerations will paradoxically make heart rate variability appear normal if measured by conventional methods. The heart rate characteristics index (HeRO score), designed as an early warning system for sepsis, incorporates measures of both decreased variability and transient decelerations.54,78,79 Descriptions of this and other sepsis early warning systems that incorporate vital sign changes are presented at the end of this review.
Changes in heart rate and HRV are not specific to sepsis and may occur in NICU patients with other conditions. Tachycardia can be due to agitation, overheating, or medications. Heart rate decelerations or bradycardias occur due to apnea of prematurity or vagal stimulation associated with a Valsalva reflex or gastroesophageal reflux. Other pathologic conditions associated with abnormal heart rate characteristics include (1) respiratory deterioration, likely mediated by lung inflammation, hypoxia, and acidosis70; (2) severe apnea with associated decelerations14; (3) infectious and inflammatory conditions other than septicemia, such as urinary tract infection80 and necrotizing enterocolitis;72 (4) surgery, due to the effect of inhaled anesthetics and inflammation from tissue trauma;14 and (5) brain injury, due to both ANS dysfunction and inflammation.67,68 Some medications are known to impact HRV in neonates. Clinical and animal studies demonstrate an increase in HRV with steroid treatment,28,77 while atropine dramatically reduces HRV due to its vagolytic effect.81 Both pain and opioid pharmacokinetics have been shown to correlate with changes in HRV.82,83,84
Respiratory and oxygenation changes in neonatal sepsis: mechanisms
Tachypnea: Neonates with sepsis may, like children and adults, present with tachypnea and respiratory distress. Rapid breathing may be due to direct pathogen toxin effects or maybe a mechanism to compensate for metabolic acidosis. Tachypnea can also be related to sepsis-associated acute lung inflammation or edema leading to hypoxia. In preterm infants, tachypnea may be present at baseline due to acute or chronic lung disease, and an acute worsening of respiratory status may occur in the absence of infection.
Apnea: Preterm infants commonly have apnea of prematurity due to immature control of breathing. An increase in central apnea is one of the most commonly cited signs of sepsis in preterm infants,1 whereas apnea in term neonates is more often a sign of neurologic dysfunction or seizure rather than sepsis.85,86 Central apnea is common both with a bacterial and viral infection, particularly with respiratory viruses such as a respiratory syncytial virus.46,87 A specific RSV glycoprotein induces substance P, which provides a mechanism for apnea with RSV disease.88
Prostaglandin E2 (PGE2) inhibits respiratory neural output and plays an important role in sepsis-associated apnea, with its production activated by cytokines.89 Neonatologists are familiar with PGE2-induced apnea in infants with congenital cardiac malformations requiring exogenous PGE2 therapy to maintain patency of the ductus arteriosus. Urinary prostaglandin metabolites have been proposed as a sepsis biomarker to detect increased production of endogenous PGE2.90 Many cytokines do not cross the blood–brain barrier, but lipopolysaccharide administration in rodents was shown to increase IL-1β and IL-6 production in the brainstem and dampened the ventilatory response to hypoxia.91 In this study, vagotomy and immunohistochemical staining provided evidence that the upregulation of cytokine production in the medulla was mediated by the vagus nerve.
Bradycardia and oxygen desaturation are often associated with apnea in premature infants. The mechanism of bradycardia during or shortly following central apnea is not fully known, but maybe mediated by hypoxia stimulating carotid chemoreceptors and by stimulation of central control of heart rate due to an interaction between central respiratory and cardiovascular signaling.92
Oxygenation: Hypoxia occurs in neonates with sepsis and may be intermittent or prolonged. Intermittent hypoxia may reflect apnea, whereas prolonged hypoxemia may be associated with pneumonia, surfactant deficiency, lung inflammation, or pulmonary edema. Hypoxia and reoxygenation increase the production of reactive oxygen species, which may lead to inflammatory tissue damage.93 An additional cause of hypoxemia in sepsis is pulmonary hypertension. Group B Streptococcal sepsis is particularly well known to cause pulmonary hypertension, at least in part due to increased thromboxane production mediated by bacterial phospholipids.94
Clinical implications of respiratory changes in sepsis
Most NICU monitors use chest impedance to report respiratory rate and the displayed values can be inaccurate due to irregular respirations and conventional monitor averaging times. This leads to a very wide range of recorded respiratory rates from less than 20 to more than 100 breaths per minute, or apparent intermittent bradypnea and tachypnea, when the true respiratory rate is usually somewhere in between if counted over 60 s. Tachypnea may be difficult to discern as a sign of sepsis in NICU patients with acute or chronic lung disease who have a high respiratory rate at baseline.
Apnea quantitation is also complicated because NICU monitors tend to have false alarms for central apnea and, even more frequently, to miss true apnea.95 The latter is due, in some cases, to a heartbeat artifact. During a prolonged pause in breathing, the monitors’ chest impedance algorithm sometimes interprets heartbeats as breaths, particularly when the heart rate slows and stroke volume increases, leading to stronger chest impedance signals.96 Researchers at the University of Virginia developed an automated detection system for central apnea that analyzes chest impedance waveforms using filters to remove motion and cardiac artifact.96 This system helped establish normative values for the number and duration of daily apnea events for preterm infants based on gestational age and chronologic age.27 Increased apnea is a common presenting sign of sepsis. Fig. 1 shows an example of apnea with an associated HR deceleration and a decline in SpO2 in a preterm infant 6 h prior to a diagnosis of sepsis. Of note, in this example, the infant is having repetitive apnea events with entrainment of HR and SpO2. Periodic breathing, with repetitive cycles of respiratory pauses and rapid breaths, occurs in all healthy term and preterm infants during sleep, and typically accounts for <10% of a 24 h period. Exaggerated periodic breathing (an increase over baseline, and occurring more than 20% of the time) has been shown to occur in some preterm infants prior to diagnosis of sepsis and NEC. In some cases, such as the example in Fig. 1, small decreases in HR with or without decreases in SpO2 accompany the short apneic pauses.97 Apnea and exaggerated periodic breathing in sepsis likely both occur due to increased endogenous prostaglandin production.
Sixty minutes of every-two-second HR (beats per minute in red), SPO2 (% in blue), and respiratory rate (RESP, breaths per minute in gray) data are shown from a preterm infant 6 h before the diagnosis of sepsis. Note the frequent small decreases in HR and SpO2 and two deeper deceleration–desaturation episodes. The inset shows 10 min of HR, SpO2, and respiratory rate during the more severe deceleration–desaturation episode. The respiratory rate shows alternating tachypnea and apnea, a characteristic of periodic breathing. The longer respiratory pause accompanied by a major decline in HR and SpO2 is an apnea–bradycardia–desaturation or “ABD” spell. Increased number or severity of apnea spells is a common presenting sign of sepsis in preterm NICU patients. Exaggerated periodic breathing can also be a sign of sepsis or respiratory viral infection in this population.
The difficulty in analyzing chest impedance waveform data to detect apnea and periodic breathing makes surrogate apnea detection algorithms appealing. One such algorithm is the cross-correlation of heart rate and SpO2, which measures the co-trending of the two signals with a set lag time. Figure 2 shows, in a preterm infant diagnosed with CONS sepsis on day 10 and NEC day 14, the trends in vital sign analytics incorporating respiratory rate, heart rate, and SpO2. The top panel shows cross-correlation, the middle panel covariance, and the bottom panel standard deviation. In a study of 1065 VLBW infants at two NICUs, the cross-correlation of HR-SpO2 performed better than all other vital sign features analyzed for predicting imminent sepsis98 and was higher in cases of confirmed sepsis compared to events in which sepsis was initially suspected but ruled out.1 A subsequent study demonstrated a correlation between excessive central apnea events or periodic breathing and high cross-correlation of HR-SpO2.99 Figure 2 demonstrates the utility of displayed vital sign analytics; changes in the variability or interaction of vital signs become more apparent.
Representative example of changes in cardiorespiratory patterns of a very low birth weight preterm infant in the days surrounding the diagnosis of coagulase-negative staphylococcus (CONS) sepsis at 10 days of age and necrotizing enterocolitis (NEC) on day 14. a Before diagnosis, there was an increase in the cross-correlation of heart rate (HR) and respiration (RESP), HR and oxygen saturation (SpO2), and RESP-SpO2. b Covariance of HR, RESP, and SpO2. c Standard deviation of hourly HR, RESP, and SpO2.
An important consideration in using respiratory deterioration or apnea spells as a sign of sepsis is that these events can also occur due to common conditions in preterm infants without infection, such as inadequate respiratory support, oxygen supplementation,100 or anemia.101 In a recent retrospective multicenter study, the most common sign cited as a reason for a sepsis workup in preterm NICU patients was increased events with apnea, bradycardia, or desaturation, but the associated diagnosis was just as often sepsis ruled out as confirmed sepsis.1 This points to the importance of knowing an infant’s prior history, including vital sign trends, and considering causes other than sepsis before embarking on testing and treatment.
Blood pressure changes in neonatal sepsis: mechanisms
Hypotension in neonatal sepsis may occur due to decreased cardiac preload, impaired myocardial contractility, or changes in systemic vascular resistance altering cardiac afterload. Intravascular volume depletion may occur following endothelial damage caused by immune cell activation, cytotoxic cytokines, or oxidative or nitrosative molecules. Cytokines such as TNFα, IL-1β have been shown to directly impact cardiac function in adult models,102 whereas in a fetal sheep model endotoxin administration induced myocardial dysfunction via toll-like receptor activation and not inflammatory cytokines.103 Cardiac output may be further compromised in sepsis-associated tachycardia, which decreases cardiac filling time and stroke volume. Poor perfusion in sepsis can also contribute to acidosis and myocardial ischemia which impact contractility leading to a vicious cycle that ends in circulatory collapse. Afterload may be decreased due to vasodilation (warm shock) or increased due to peripheral vasoconstriction (cold shock), the latter being more common in neonatal sepsis.36
Other endogenous mediators also contribute to hypotension in sepsis. Nitric oxide, produced by inducible nitric oxide synthase, triggered by endothelial nitric oxide synthase, facilitates septic shock. Prostanoids (prostaglandins and thromboxanes) increase during sepsis and have potent vasoactive properties that contribute to hypotension. Endogenous glucocorticoids also regulate blood pressure, and preterm neonates with relative adrenal insufficiency often develop low blood pressure in the setting of severe infection.
Variability of blood pressure may also be impacted in neonatal sepsis. One study reported repetitive waves of blood pressure increase by ten or more mmHg in NICU patients with sepsis, associated with surges of norepinephrine.104 In another study, the occurrence of similar repetitive waves of BP increase was present in some but not all preterm NICU patients treated with vasopressors for hypotension, and the absence of these BP surges was associated with vasopressor resistance.105
Clinical implications of blood pressure changes
Hypotension is uncommon as a presenting sign of neonatal sepsis but, if present, indicates serious illness and a high risk for morbidity and mortality. Tachycardia, poor perfusion, or oliguria may precede hypotension, and identifying and treating these changes before progression to shock may avert ischemic organ damage. Dopamine is the most common initial vasoactive medication used in the NICU, but alternative medications may be more effective for extremely preterm infants. These infants have low stores and production of catecholamines and therefore may not respond to dopamine. Vasopressin treatment has been shown to be effective for some premature infants with sepsis and refractory hypotension.106,107,108 Hydrocortisone treatment often raises blood pressure and has been reported to reduce the total dose and duration of vasoactive medication use in premature infants,109 especially those with lower pre-treatment endogenous cortisol levels.110
As medical technology advances, new “vital signs” become available for monitoring. Near-infrared spectroscopy (NIRS) technology was developed in the 1980s and has since been adapted for use in neonates. NIRS measures regional tissue oxygenation,111 and limited studies have reported on changes in NIRS measurements during sepsis. A pilot study showed no change in cerebral NIRS during sepsis, but most cases were caused by CONS and did not result in severe sepsis.112 Splanchnic oxygenation has been shown to both decrease and increase near the diagnosis of NEC.113 Its variability may also contain predictive information as it detects a change in the body’s ability to autoregulate perfusion to vital organs.114,115
Temperature instability in neonatal sepsis: mechanisms
Neonates with sepsis, especially those born preterm, develop hypothermia more often than adults and children, who present more often with fever.116,117 A notable exception is a neonatal infection with Herpes Simplex Virus, which commonly presents with fever in the first days to weeks after birth. Whether infection leads to fever or hypothermia is due to the relative production of endogenous pyrogens and cryogens. Newborn infants have elevated levels of arginine vasopressin,118 an antipyretic, and this may account for fever being uncommon in neonatal sepsis. Temperature response may also vary by the pathogen. In a rodent model of neonatal sepsis, the administration of Gram-negative lipopolysaccharide-induced hypothermia, while Gram-positive toxins did not.119 The major fever-inducing cytokines are IL-6 and IL-1β,120 whereas TNF-α produces initial hypothermia followed by fever and correlates with increased sepsis mortality.121
Prostaglandins, particularly PGE2, play a key role in the induction of fever during inflammation. Immune and endothelial cells in the brain release PGE2, which acts on the hypothalamus to increase the body’s thermostat.122,123 Immaturity of thermoregulatory mechanisms may also play a role in sepsis-associated hypothermia in neonates. High body surface area for heat loss, decreased thyroid hormone levels, and less brown fat thermogenesis increases the risk for hypothermia. Sepsis may cause additional derangement, not only with the production of endogenous cryogenic substances but also with circulatory and metabolic changes leading to heat loss.
Clinical implications of temperature instability
In many cases, increased or decreased body temperature in neonates is due to exogenous factors and not sepsis. For example, over bundling of term neonates can result in elevated temperature, and inadequate provision of external heat to preterm neonates leads to hypothermia. Nonetheless, the abnormal temperature in a neonate should prompt close observation and consideration of infection. In the outpatient setting, a study of mostly term infants presenting to the Emergency Department with hypothermia showed that 15% had a serious bacterial infection.124 In the NICU, incubators maintain normothermia using servo-controlled air temperature. Thus, temperature derangements may be masked, while fluctuations in the incubator temperature could indicate sepsis-related temperature instability.
The difference between core and peripheral temperature has also been studied as an indicator of sepsis. Peripheral vasoconstriction redirects blood and heat to the vital organs and maintains core temperature while lowering the temperature of the extremities. Dail and colleagues have studied the continuous measurement of the difference between core and peripheral temperature as an indicator of sepsis.125,126 Observational studies show this temperature difference occurs more often in extremely preterm infants, likely due to immature autonomic control.125 An ongoing clinical trial is evaluating continuous core to peripheral temperature difference monitoring to predict infection in premature infants.127
Fever and hypothermia are part of normal host defense mechanisms, and both are double-edged swords with potential for both benefit and harm. Fever augments leukocyte function and other aspects of host defense, but also raises the metabolic rate and can lead to collateral damage to tissues such as the lung and brain.128,129 Hypothermia, conversely, is generally thought of as a maladaptive response to infection reflecting greater severity of illness,116,130 yet it also lowers metabolic rate and has tissue-protective properties in some settings.131
Cholinergic anti-inflammatory response in sepsis
While the body sets inflammatory cascades in motion to fight infection, it also increases anti-inflammatory cytokines and neural pathways to maintain homeostasis. The cholinergic anti-inflammatory response, described by Tracey and others, involves the interaction between inflammatory molecules and neural circuits that triggers feedback loops via the vagus nerve to curb the inflammatory response.7,132 Acetylcholine binds to nicotinic cholinergic receptors on macrophages and other immune cells and inhibits the release of TNF-α and other pro-inflammatory cytokines.
The cholinergic anti-inflammatory pathway also triggers the production of anti-inflammatory cytokines such as IL-10 and TGF-β and activation of the hypothalamic–pituitary–adrenal axis to produce endogenous steroids that further modulate the systemic inflammatory response.6,133 Blocking the cholinergic anti-inflammatory response increases sepsis lethality in animal models, whereas vagus nerve stimulation or pharmacologic activation of the response through nicotine agonists has beneficial effects.53,134 For septic neonates, repetitive brief heart rate decelerations may be due, at least in part, to vagus nerve firing as a manifestation of this protective parasympathetic reflex.54
Scores and systems for neonatal sepsis risk assessment
To facilitate earlier diagnosis and treatment of neonatal sepsis, a number of groups have developed risk scores, clinical decision support tools, or predictive models that incorporate vital sign changes. In the era of big data research with the ability to extract and analyze large datasets from the electronic health record, advanced analytics will continue to be developed, refined, and implemented to improve outcomes for NICU patients. Here, we review some, but not all, warning systems and scores for neonatal sepsis, most in the research and development phase.
The first commercially available early warning system for neonatal sepsis is the HeRO (Heart Rate Observation) monitor (Medical Predictive Science Corporation, Charlottesville, VA). Researchers developed a mathematical algorithm to quantify what experienced clinicians noticed while reviewing electrocardiogram data of preterm infants with sepsis: decreased HR variability, punctuated by transient HR decelerations. The algorithm detects these features by measuring beat-to-beat HR variability, sample entropy,135 and skewness as the ratio of accelerations to decelerations.136 The HR characteristics index (HeRO score) was developed as the fold-increased risk, which means that it compares the predicted risk of sepsis in the next 24 h to the average risk for all VLBW infants at all times. Display of this score reduced all-cause mortality by 22%137 and sepsis-associated mortality by 40%138 in a randomized clinical trial of 3003 VLBW infants in nine NICUs.
Decreased activity or lethargy often prompts concern for sepsis but is subjective. One group developed a continuous measure from ECG waveforms to continuously quantify infant motion for early sepsis detection. This algorithm, called the Signal Instability Index (SII) estimates the extent and duration of movement every second based on the previous ten seconds of ECG data.139 In a study testing the predictive performance of the SII and individual heart rate and respiratory signal features, measures of the SII did not perform as well as heart rate characteristics,140 but combining this continuous index of movement could potentially add information to heart rate predictive monitoring in the clinical setting.
Another neonatal sepsis alert system is RALIS, in which nurses enter multiple vital signs every 2 h, including HR, respiratory rate, temperature, and bradycardia and oxygen desaturation events. A score >5 prompts clinicians to evaluate for possible sepsis.61,62,63 A Neonatal Necrotizing Enterocolitis Early Detection Score (NeoNEEDs) was developed in a single-center retrospective cohort and prospectively validated. Cardiorespiratory instability was the most common early presenting sign of NEC, and a high score was used as a trigger for clinicians to evaluate an infant and obtain an abdominal radiograph.141
While predictive algorithms based on continuous monitoring data can provide early sepsis warning, models incorporating abnormal physiology in the perinatal period can predict risk of later morbidities by stratifying based on illness severity. Predicting risk of later deterioration from sepsis or NEC could identify preterm infants requiring enhanced vigilance and allows for risk stratification in research for an adjusted comparison of outcomes. In a small study of HR, RR, and SpO2 data from the first 3 h after the birth of preterm infants, a Physiscore was developed that predicted a variety of later adverse events, including sepsis.142 An elevated Physiscore was also associated with evidence of intrauterine infection on histologic examination of the placentas.143 Neonatal illness severity scores, such as the Clinical Risk Indicator for Babies (CRIB)144 and Score for Acute Neonatal Physiology (SNAP)145 predict mortality based on data collected within the first 6–12 h after birth, but perform less reliably when calculated serially for early warning of illness.146
A weakness in neonatal sepsis predictive algorithms is the lack of a consensus definition that incorporates illness severity.15 The Sequential Organ Failure Assessment (SOFA) score for adults was adapted for pediatric147 and neonatal patients148 to assess sepsis-associated mortality. The nSOFA score, validated in a multicenter cohort149 incorporates vital sign and laboratory parameters and represents a step toward defining neonatal sepsis according to illness severity.
Advances in bioinformatics and data science have brought an influx of studies developing machine learning models using large datasets extracted from electronic health records. The body of literature on machine learning for adult sepsis is large,150 and growing for pediatric and neonatal sepsis.151,152,153 Adults and children with sepsis have considerable variability in age and comorbidities that influence model training and performance154 and for preterm infants risk correlates strongly with gestational age and comorbidities.43 One goal of predictive analytics is to build a model that adds to what the clinician already knows, or one that detects abnormal physiology and not just clinician-initiated data from the electronic health record.155
Conclusion
Changes in vital signs can signal sepsis, and proper use of sepsis early warning systems in the ICU can improve outcomes through earlier treatments leading to more rapid resolution of cardiorespiratory instability and systemic inflammation.156 While antibiotic therapy can be life-saving for true infections, overuse of antibiotics in the absence of infection has many detrimental effects and should be avoided. It is critically important that any sepsis early warning system alert or score should be interpreted in the context of other clinical and vital sign changes, as well as laboratory testing when appropriate. Many vital sign changes or patterns occur in response to conditions other than sepsis including clinical interventions and medications. Therefore, through a combination of enhanced clinical vigilance and informed use of risk scores, NICU patients can benefit from “right timing” antibiotics leading to better outcomes for septic neonates.
References
Sullivan, B. et al. Clinical and vital sign changes associated with late-onset sepsis in very low birth weight infants at 3 NICUs. J. Neonatal Perinatal Med. 1–9 (2021).
Griffin, M. P., Lake, D. E., O’Shea, T. M. & Moorman, J. R. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatr. Res. 61, 222–227 (2007).
Mayampurath, A., Jani, P., Dai, Y., Gibbons, R., Edelson, D. & Churpek, M. A vital sign-based model to predict clinical deterioration in hospitalized children. Pediatr. Crit. Care Med. 21, 820–826 (2020).
Kaukonen, K.-M., Bailey, M., Pilcher, D., Cooper, D. J. & Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 372, 1629–1638 (2015).
Coggins, S., Harris, M. C., Grundmeier, R., Kalb, E., Nawab, U. & Srinivasan, L. Performance of pediatric systemic inflammatory response syndrome and organ dysfunction criteria in late-onset sepsis in a quaternary neonatal intensive care unit: a case-control study. J. Pediatr. 219, 133–139.e1 (2020).
Tracey, K. J. Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428 (2009).
Tracey, K. J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 117, 289–296 (2007).
Moorman, J. R., Lake, D. E. & Ivanov, P. C. Early detection of sepsis–a role for network physiology? Crit. Care Med. 44, e312–e313 (2016).
Bartsch, R. P., Liu, K. K. L., Bashan, A. & Ivanov, P. C. Network physiology: how organ systems dynamically interact. PLoS ONE 10, e0142143 (2015).
Eckberg, D. L. Human sinus arrhythmia as an index of vagal cardiac outflow. J. Appl Physiol. 54, 961–966 (1983).
Clark, M. T. et al. Breath-by-breath analysis of cardiorespiratory interaction for quantifying developmental maturity in premature infants. J. Appl Physiol. 112, 859–867 (2012).
Mulkey, S. B. et al. Autonomic nervous system maturation in the premature extrauterine milieu. Pediatr. Res. 89, 863–868 (2021).
Schlatterer, S. D. et al. Autonomic development in preterm infants is associated with morbidity of prematurity. Pediatr. Res. (2021).
Sullivan, B. A. & Fairchild, K. D. Predictive monitoring for sepsis and necrotizing enterocolitis to prevent shock. Semin. Fetal Neonatal Med. 20, 255–261 (2015).
Wynn, J. L. & Polin, R. A. Progress in the management of neonatal sepsis: the importance of a consensus definition. Pediatr. Res. 83, 13–15 (2018).
Goldstein, B., Giroir, B. & Randolph, A. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 6, 2–8 (2005).
Hofer, N., Zacharias, E., Müller, W. & Resch, B. Performance of the definitions of the systemic inflammatory response syndrome and sepsis in neonates. J. Perinat. Med. 40, 587–590 (2012).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016).
Wynn, J. L. et al. Postnatal age is a critical determinant of the neonatal host response to sepsis. Mol. Med. 21, 496–504 (2015).
Esposito, S. et al. Genetic polymorphisms and sepsis in premature neonates. PLoS ONE 9, e101248 (2014).
Hotchkiss, R. S., Moldawer, L. L., Opal, S. M., Reinhart, K., Turnbull, I. R. & Vincent, J.-L. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2, 16045 (2016).
Casadevall, A. & Pirofski, L. Host-pathogen interactions: the attributes of virulence. J. Infect. Dis. 184, 337–344 (2001).
Wynn, J., Cornell, T. T., Wong, H. R., Shanley, T. P. & Wheeler, D. S. The host response to sepsis and developmental impact. Pediatrics 125, 1031–1041 (2010).
Alonzo, C. J., Nagraj, V. P., Zschaebitz, J. V., Lake, D. E., Moorman, J. R. & Spaeder, M. C. Heart rate ranges in premature neonates using high resolution physiologic data. J. Perinatol. 38, 1242–1245 (2018).
Hegyi, T. et al. Blood pressure ranges in premature infants. I. The first hours of life. J. Pediatr. 124, 627–633 (1994).
Hegyi, T. et al. Blood pressure ranges in premature infants: II. The first week of life. Pediatrics 97, 336–342 (1996).
Fairchild, K. et al. Clinical associations of immature breathing in preterm infants: part 1-central apnea. Pediatr. Res. 80, 21–27 (2016).
Alonzo, C. J. & Fairchild, K. D. Dexamethasone effect on heart rate variability in preterm infants on mechanical ventilation. J. Neonatal Perinat. Med. 10, 425–430 (2017).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Sjoding, M. W., Dickson, R. P., Iwashyna, T. J., Gay, S. E. & Valley, T. S. Racial bias in pulse oximetry measurement. N. Engl. J. Med. 383, 2477–2478 (2020).
Khera, A. et al. Race and gender differences in C-reactive protein levels. J. Am. Coll. Cardiol. 46, 464–469 (2005).
Koenig, J. & Thayer, J. F. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci. Biobehav. Rev. 64, 288–310 (2016).
Sampath, V. et al. Toll-like receptor genetic variants are associated with Gram-negative infections in VLBW infants. J. Perinatol. 33, 772–777 (2013).
Abu-Maziad, A. et al. Role of polymorphic variants as genetic modulators of infection in neonatal sepsis. Pediatr. Res. 68, 323–329 (2010).
Schelonka, R. L. et al. T cell cytokines and the risk of blood stream infection in extremely low birth weight infants. Cytokine 53, 249–255 (2011).
Wynn, J. L. & Wong, H. R. Pathophysiology and treatment of septic shock in neonates. Clin. Perinatol. 37, 439–479 (2010).
Craft, A. & Finer, N. Nosocomial coagulase negative staphylococcal (CoNS) catheter-related sepsis in preterm infants: definition, diagnosis, prophylaxis, and prevention. J. Perinatol. 21, 186–192 (2001).
Cantey, J. B., Anderson, K. R., Kalagiri, R. R. & Mallett, L. H. Morbidity and mortality of coagulase-negative staphylococcal sepsis in very-low-birth-weight infants. World J. Pediatr. 14, 269–273 (2018).
Raynor, L. L., Saucerman, J. J., Akinola, M. O., Lake, D. E., Moorman, J. R. & Fairchild, K. D. Cytokine screening identifies NICU patients with Gram-negative bacteremia. Pediatr. Res. 71, 261–266 (2012).
Rønnestad, A., Abrahamsen, T. G., Gaustad, P. & Finne, P. H. C-reactive protein (CRP) response patterns in neonatal septicaemia. APMIS 107, 593–600 (1999).
Coggins, S. A. et al. Use of a computerized C-reactive protein (CRP) based sepsis evaluation in very low birth weight (VLBW) infants: a five-year experience. PLoS ONE 8, e78602 (2013).
Brown, J. V. E., Meader, N., Cleminson, J. & McGuire, W. C-reactive protein for diagnosing late-onset infection in newborn infants. Cochrane Database Syst. Rev. 1, CD012126 (2019).
Stoll, B. J. et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110, 285–291 (2002).
Parlato, M. & Cavaillon, J.-M. Host response biomarkers in the diagnosis of sepsis: a general overview. Methods Mol. Biol. 1237, 149–211 (2015).
Dickson, K. & Lehmann, C. Inflammatory response to different toxins in experimental sepsis models. Int. J. Mol. Sci. 20, 4341 (2019).
Kidszun, A. et al. Viral infections in neonates with suspected late-onset bacterial sepsis—a prospective cohort study. Am. J. Perinatol. 34, 1–7 (2017).
Ng, P. C. et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch. Dis. Child Fetal Neonatal Ed. 88, F209–F213 (2003).
Idborg, H. et al. Evaluation of urinary prostaglandin E2 metabolite as a biomarker in infants with fever due to viral infection. Prostaglandins Leukot. Ess. Fat. Acids 91, 269–275 (2014).
Berthoud, H. R. & Neuhuber, W. L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 85, 1–17 (2000).
Carrara, M., Ferrario, M., Bollen Pinto, B. & Herpain, A. The autonomic nervous system in septic shock and its role as a future therapeutic target: a narrative review. Ann. Intensive Care. 11, 80 (2021).
Marijianowski, M. M., van der Loos, C. M., Mohrschladt, M. F. & Becker, A. E. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J. Am. Coll. Cardiol. 23, 1204–1208 (1994).
Habimana, R., Choi, I., Cho, H. J., Kim, D., Lee, K. & Jeong, I. Sepsis-induced cardiac dysfunction: a review of pathophysiology. Acute Crit. Care. 35, 57–66 (2020).
Borovikova, L. V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000).
Fairchild, K. D., Srinivasan, V., Moorman, J. R., Gaykema, R. P. A. & Goehler, L. E. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R330–R339 (2011).
Herlenius, E. An inflammatory pathway to apnea and autonomic dysregulation. Respir. Physiol. Neurobiol. 178, 449–457 (2011).
Hofstetter, A. O., Saha, S., Siljehav, V., Jakobsson, P.-J. & Herlenius, E. The induced prostaglandin E2 pathway is a key regulator of the respiratory response to infection and hypoxia in neonates. Proc. Natl Acad. Sci. USA 104, 9894–9899 (2007).
Wee, B. Y. H., Lee, J. H., Mok, Y. H. & Chong, S.-L. A narrative review of heart rate and variability in sepsis. Ann. Transl. Med. 8, 768 (2020).
Ahmad, S., Tejuja, A., Newman, K. D., Zarychanski, R. & Seely, A. J. Clinical review: a review and analysis of heart rate variability and the diagnosis and prognosis of infection. Crit. Care. 13, 232 (2009).
Taylor, J. A. & Eckberg, D. L. Fundamental relations between short-term RR interval and arterial pressure oscillations in humans. Circulation 93, 1527–1532 (1996).
Thompson, C. R., Brown, J. S., Gee, H. & Taylor, E. W. Heart rate variability in healthy term newborns: the contribution of respiratory sinus arrhythmia. Early Hum. Dev. 31, 217–228 (1993).
Hathorn, M. K. Respiratory sinus arrhythmia in new-born infants. J. Physiol. 385, 1–12 (1987).
Ori, Z., Monir, G., Weiss, J., Sayhouni, X. & Singer, D. H. Heart rate variability: frequency domain analysis. Cardiol. Clin. 10, 499–533 (1992).
Chang, K. L., Monahan, K. J., Griffin, M. P., Lake, D. & Moorman, J. R. Comparison and clinical application of frequency domain methods in analysis of neonatal heart rate time series. Ann. Biomed. Eng. 29, 764–774 (2001).
Ikeda, T. et al. Fetal heart rate patterns in postasphyxiated fetal lambs with brain damage. Am. J. Obstet. Gynecol. 179, 1329–1337 (1998).
Chung, D. Y., Sim, Y. B., Park, K. T., Yi, S. H., Shin, J. C. & Kim, S. P. Spectral analysis of fetal heart rate variability as a predictor of intrapartum fetal distress. Int J. Gynaecol. Obstet. 73, 109–116 (2001).
Salafia, C. M., Mangam, H. E., Weigl, C. A., Foye, G. J. & Silberman, L. Abnormal fetal heart rate patterns and placental inflammation. Am. J. Obstet. Gynecol. 160, 140–147 (1989).
Fairchild, K. D. et al. Abnormal heart rate characteristics are associated with abnormal neuroimaging and outcomes in extremely low birth weight infants. J. Perinatol. 34, 375–379 (2014).
Vergales, B. D. et al. Depressed heart rate variability is associated with abnormal EEG, MRI, and death in neonates with hypoxic ischemic encephalopathy. Am. J. Perinatol. 31, 855–862 (2014).
Thiriez, G. et al. Altered autonomic control in preterm newborns with impaired neurological outcomes. Clin. Auton. Res. 25, 233–242 (2015).
Sullivan, B. A., Grice, S. M., Lake, D. E., Moorman, J. R. & Fairchild, K. D. Infection and other clinical correlates of abnormal heart rate characteristics in preterm infants. J. Pediatr. 164, 775–780 (2014).
Griffin, M. P., Lake, D. E., Bissonette, E. A., Harrell, F. E., O’Shea, T. M. & Moorman, J. R. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics 116, 1070–1074 (2005).
Stone, M. L. et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. J. Perinatol. 33, 847–850 (2013).
Doheny, K. K. et al. Diminished vagal tone is a predictive biomarker of necrotizing enterocolitis-risk in preterm infants. Neurogastroenterol. Motil. 26, 832–840 (2014).
Weitkamp, J.-H. et al. Meningitis, urinary tract, and bloodstream infections in very low birth weight infants enrolled in a heart rate characteristics monitoring trial. Pediatr. Res. 87, 1226–1230 (2020).
Griffin, M. P., Scollan, D. F. & Moorman, J. R. The dynamic range of neonatal heart rate variability. J. Cardiovasc. Electrophysiol. 5, 112–124 (1994).
Huston, J. M. & Tracey, K. J. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J. Intern Med. 269, 45–53 (2011).
Fairchild, K. D. et al. Endotoxin depresses heart rate variability in mice: cytokine and steroid effects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1019–R1027 (2009).
Griffin, M. P., O’Shea, T. M., Bissonette, E. A., Harrell, F. E., Lake, D. E. & Moorman, J. R. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr. Res. 53, 920–926 (2003).
Fairchild, K. D. & O’Shea, T. M. Heart rate characteristics: physiomarkers for detection of late-onset neonatal sepsis. Clin. Perinatol. 37, 581–598 (2010).
Aviles-Otero, N. et al. Urinary tract infections in very low birthweight infants: A two-center analysis of microbiology, imaging and heart rate characteristics. J. Neonatal Perinatal. Med. 14, 269–276 (2020).
Andriessen, P., Janssen, B. J. A., Berendsen, R. C. M., Oetomo, S. B., Wijn, P. F. F. & Blanco, C. E. Cardiovascular autonomic regulation in preterm infants: the effect of atropine. Pediatr. Res. 56, 939–946 (2004).
Bressan, N., McGregor, C., Smith, K., Lecce, L. & James, A. Heart rate variability as an indicator for morphine pharmacokinetics and pharmacodynamics in critically ill newborn infants. Conf. Proc. IEEE Eng. Med Biol. Soc. 2014, 5719–5722 (2014).
Faye, P. M. et al. Newborn infant pain assessment using heart rate variability analysis. Clin. J. Pain. 26, 777–782 (2010).
Cong, X., Ludington-Hoe, S. M., McCain, G. & Fu, P. Kangaroo care modifies preterm infant heart rate variability in response to heel stick pain: pilot study. Early Hum. Dev. 85, 561–567 (2009).
Sirsi, D., Nadiminti, L., Packard, M. A., Engel, M. & Solomon, G. E. Apneic seizures: a sign of temporal lobe hemorrhage in full-term neonates. Pediatr. Neurol. 37, 366–370 (2007).
Patrinos, M. E. & Martin, R. J. Apnea in the term infant. Semin Fetal Neonatal Med. 22, 240–244 (2017).
Ralston, S. & Hill, V. Incidence of apnea in infants hospitalized with respiratory syncytial virus bronchiolitis: a systematic review. J. Pediatr. 155, 728–733 (2009).
Tripp, R. A., Dakhama, A., Jones, L. P., Barskey, A., Gelfand, E. W. & Anderson, L. J. The G glycoprotein of respiratory syncytial virus depresses respiratory rates through the CX3C motif and substance P. J. Virol. 77, 6580–6584 (2003).
Siljehav, V., Hofstetter, A. M., Leifsdottir, K. & Herlenius, E. Prostaglandin E2 mediates cardiorespiratory disturbances during infection in neonates. J. Pediatr. 167, 1207–13.e3 (2015).
Hamrin, J. et al. Urinary PGE2 metabolite levels in hospitalised infants with infections compared to age-matched controls. Acta Paediatr. 108, 1879–1886 (2019).
Balan, K. V., Kc, P., Hoxha, Z., Mayer, C. A., Wilson, C. G. & Martin, R. J. Vagal afferents modulate cytokine-mediated respiratory control at the neonatal medulla oblongata. Respir. Physiol. Neurobiol. 178, 458–464 (2011).
Abu-Shaweesh, J. M. & Martin, R. J. Neonatal apnea: what’s new? Pediatr. Pulmonol. 43, 937–944 (2008).
Di Fiore, J. M. & Vento, M. Intermittent hypoxemia and oxidative stress in preterm infants. Respir. Physiol. Neurobiol. 266, 121–129 (2019).
Curtis, J., Kim, G., Wehr, N. B. & Levine, R. L. Group B streptococcal phospholipid causes pulmonary hypertension. Proc. Natl Acad. Sci. USA 100, 5087–5090 (2003).
Vergales, B. D. et al. Accurate automated apnea analysis in preterm infants. Am. J. Perinatol. 31, 157–162 (2014).
Lee, H. et al. A new algorithm for detecting central apnea in neonates. Physiol. Meas. 33, 1–17 (2012).
Patel, M. et al. Clinical associations with immature breathing in preterm infants: part 2-periodic breathing. Pediatr. Res. 80, 28–34 (2016).
Fairchild, K. D. et al. Vital signs and their cross-correlation in sepsis and NEC: a study of 1,065 very-low-birth-weight infants in two NICUs. Pediatr. Res. 81, 315–321 (2017).
Fairchild, K. D. & Lake, D. E. Cross-correlation of heart rate and oxygen saturation in very low birthweight infants: association with apnea and adverse events. Am. J. Perinatol. 35, 463–469 (2018).
Warburton, A., Monga, R., Sampath, V. & Kumar, N. Continuous pulse oximetry and respiratory rate trends predict short-term respiratory and growth outcomes in premature infants. Pediatr. Res. 85, 494–501 (2019).
Zagol, K. et al. Anemia, apnea of prematurity, and blood transfusions. J. Pediatr. 161, 417–421.e1 (2012).
Hofmann, U. et al. The proinflammatory cytokines TNF-alpha and IL-1 beta impair economy of contraction in human myocardium. Cytokine 39, 157–162 (2007).
Seehase, M. et al. Myocardial response in preterm fetal sheep exposed to systemic endotoxinaemia. Pediatr. Res. 70, 242–246 (2011).
Wefers, B., Cunningham, S., Stephen, R. & McIntosh, N. Neonatal blood pressure waves are associated with surges of systemic noradrenaline. Arch. Dis. Child Fetal Neonatal Ed. 94, F149–F151 (2009).
Vesoulis, Z. A., Hao, J., McPherson, C., El Ters, N. M. & Mathur, A. M. Low-frequency blood pressure oscillations and inotrope treatment failure in premature infants. J. Appl Physiol. 123, 55–61 (2017).
Bidegain, M., Greenberg, R., Simmons, C., Dang, C., Cotten, C. M. & Smith, P. B. Vasopressin for refractory hypotension in extremely low birth weight infants. J. Pediatr. 157, 502–504 (2010).
Shivanna, B., Rios, D., Rossano, J., Fernandes, C. J. & Pammi, M. Vasopressin and its analogues for the treatment of refractory hypotension in neonates. Cochrane Database Syst. Rev. 3, CD009171 (2013).
Meyer, S., Löffler, G., Polcher, T., Gottschling, S. & Gortner, L. Vasopressin in catecholamine-resistant septic and cardiogenic shock in very-low-birthweight infants. Acta Paediatr. 95, 1309–1312 (2006).
Ng, P. C. et al. A double-blind, randomized, controlled study of a “stress dose” of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics 117, 367–375 (2006).
Peeples, E. S. An evaluation of hydrocortisone dosing for neonatal refractory hypotension. J. Perinatol. 37, 943–946 (2017).
Vesoulis, Z. A., Mintzer, J. P. & Chock, V. Y. Neonatal NIRS monitoring: recommendations for data capture and review of analytics. J. Perinatol. 41, 675–688 (2021).
Zonnenberg, I. A., Dijk, J., van, Dungen, F. A. M., van den, Vermeulen, R. J. & Weissenbruch, M. Mvan The prognostic value of NIRS in preterm infants with (suspected) late-onset sepsis in relation to long term outcome: a pilot study. PLoS ONE 14, e0220044 (2019).
Martini, S. & Corvaglia, L. Splanchnic NIRS monitoring in neonatal care: rationale, current applications and future perspectives. J. Perinatol. 38, 431–443 (2018).
Spaeder, M. C., Klugman, D., Skurow-Todd, K., Glass, P., Jonas, R. A. & Donofrio, M. T. Perioperative near-infrared spectroscopy monitoring in neonates with congenital heart disease: relationship of cerebral tissue oxygenation index variability with neurodevelopmental outcome. Pediatr. Crit. Care Med. 18, 213–218 (2017).
Wong, F. Y. et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008).
Ahmad, M. S., Ali, N., Mehboob, N., Mehmood, R., Ahmad, M. & Wahid, A. Temperature on admission among cases of neonatal sepsis and its association with mortality. J. Pak. Med Assoc. 66, 1303–1306 (2016).
Hofer, N., Müller, W. & Resch, B. Neonates presenting with temperature symptoms: role in the diagnosis of early onset sepsis. Pediatr. Int. 54, 486–490 (2012).
Pittman, Q. J., Chen, X., Mouihate, A., Hirasawa, M. & Martin, S. Arginine vasopressin, fever and temperature regulation. Prog. Brain Res. 119, 383–392 (1998).
Falck, M. et al. Neonatal systemic inflammation induces inflammatory reactions and brain apoptosis in a pathogen-specific manner. Neonatology 113, 212–220 (2018).
Leon, L. R. Hypothermia in systemic inflammation: role of cytokines. Front. Biosci. 9, 1877–1888 (2004).
Leon, L. R., White, A. A. & Kluger, M. J. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am. J. Physiol. 275, R269–R277 (1998).
Engblom, D. et al. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat. Neurosci. 6, 1137–1138 (2003).
Garami, A., Steiner, A. A. & Romanovsky, A. A. Fever and hypothermia in systemic inflammation. Handb. Clin. Neurol. 157, 565–597 (2018).
Ramgopal, S., Noorbakhsh, K. A., Pruitt, C. M., Aronson, P. L., Alpern, E. R. & Hickey, R. W. Outcomes of young infants with hypothermia evaluated in the emergency department. J. Pediatr. 221, 132–137.e2 (2020).
Knobel-Dail, R. B., Sloane, R., Holditch-Davis, D. & Tanaka, D. T. Negative temperature differential in preterm infants less than 29 weeks gestational age: associations with infection and maternal smoking. Nurs. Res. 66, 442–453 (2017).
Knobel, R. B., Holditch-Davis, D., Schwartz, T. A. & Wimmer, J. E. Extremely low birth weight preterm infants lack vasomotor response in relationship to cold body temperatures at birth. J. Perinatol. 29, 814–821 (2009).
Dail, R. B. et al. Predicting infection in very preterm infants: a study protocol. Nurs. Res. 70, 142–149 (2020).
Hasday, J. D., Fairchild, K. D. & Shanholtz, C. The role of fever in the infected host. Microbes Infect. 2, 1891–1904 (2000).
Jiang, N. M. et al. Febrile illness and pro-inflammatory cytokines are associated with lower neurodevelopmental scores in Bangladeshi infants living in poverty. BMC Pediatr. 14, 50 (2014).
Mathur, N. B., Krishnamurthy, S. & Mishra, T. K. Evaluation of WHO classification of hypothermia in sick extramural neonates as predictor of fatality. J. Trop. Pediatr. 51, 341–345 (2005).
Zhu, C. et al. Post-ischemic hypothermia-induced tissue protection and diminished apoptosis after neonatal cerebral hypoxia-ischemia. Brain Res. 996, 67–75 (2004).
Borovikova, L. V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000).
Kressel, A. M. et al. Identification of a brainstem locus that inhibits tumor necrosis factor. Proc. Natl Acad. Sci. USA 117, 29803–29810 (2020).
Bernik, T. R. et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J. Exp. Med. 195, 781–788 (2002).
Lake, D. E., Richman, J. S., Griffin, M. P. & Moorman, J. R. Sample entropy analysis of neonatal heart rate variability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R789–R797 (2002).
Kovatchev, B. P., Farhy, L. S., Cao, H., Griffin, M. P., Lake, D. E. & Moorman, J. R. Sample asymmetry analysis of heart rate characteristics with application to neonatal sepsis and systemic inflammatory response syndrome. Pediatr. Res. 54, 892–898 (2003).
Moorman, J. R. et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J. Pediatr. 159, 900–6.e1 (2011).
Fairchild, K. D. et al. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatr. Res. 74, 570–575 (2013).
Joshi, R. et al. A ballistographic approach for continuous and non-obtrusive monitoring of movement in neonates. IEEE J. Transl. Eng. Health Med. 6, 2700809 (2018).
Joshi, R., Kommers, D., Oosterwijk, L., Feijs, L., van Pul, C. & Andriessen, P. Predicting neonatal sepsis using features of heart rate variability, respiratory characteristics, and ECG-derived estimates of infant motion. IEEE J. Biomed. Health Inform. 24, 681–692 (2020).
Fox, J. R., Thacker, L. R. & Hendricks-Muñoz, K. D. Early detection tool of intestinal dysfunction: impact on necrotizing enterocolitis severity. Am. J. Perinatol. 32, 927–932 (2015).
Saria, S., Rajani, A. K., Gould, J., Koller, D. & Penn, A. A. Integration of early physiological responses predicts later illness severity in preterm infants. Sci. Transl. Med. 2, 48ra65 (2010).
Chisholm, K. M. et al. Correlation of preterm infant illness severity with placental histology. Placenta 39, 61–69 (2016).
International Neonatal Network. The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. Lancet 342, 193–198 (1993).
Richardson, D. K., Corcoran, J. D., Escobar, G. J. & Lee, S. K. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J. Pediatr. 138, 92–100 (2001).
Fowlie, P. W., Gould, C. R., Tarnow-Mordi, W. O. & Strang, D. Measurement properties of the clinical risk index for babies-reliabilty, validity beyond the first 12 h, and responsiveness over 7 days. Crit. Care Med. 26, 163–168 (1998).
Matics, T. J. & Sanchez-Pinto, L. N. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. 171, e172352 (2017).
Wynn, J. L. & Polin, R. A. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr. Res. 88, 85–90 (2020).
Fleiss, N. et al. Evaluation of the neonatal sequential organ failure assessment and mortality risk in preterm infants with late-onset infection. JAMA Netw. Open. 4, e2036518 (2021).
Kausch, S. L., Moorman, J. R., Lake, D. E. & Keim-Malpass, J. Physiological machine learning models for prediction of sepsis in hospitalized adults: an integrative review. Intensive Crit. Care Nurs. 65, 103035 (2021).
Song, W., Jung, S. Y., Baek, H., Choi, C. W., Jung, Y. H. & Yoo, S. A predictive model based on machine learning for the early detection of late-onset neonatal sepsis: development and observational study. JMIR Med. Inform. 8, e15965 (2020).
Mani, S. et al. Medical decision support using machine learning for early detection of late-onset neonatal sepsis. J. Am. Med Inf. Assoc. 21, 326–336 (2014).
Masino, A. J. et al. Machine learning models for early sepsis recognition in the neonatal intensive care unit using readily available electronic health record data. PLoS ONE 14, e0212665 (2019).
Spaeder, M. C. et al. Predictive analytics in the pediatric intensive care unit for early identification of sepsis: capturing the context of age. Pediatr. Res. 86, 655–661 (2019).
Beaulieu-Jones, B. K. et al. Machine learning for patient risk stratification: standing on, or looking over, the shoulders of clinicians? npj Digital Med. 4, 62 (2021).
Kumar, N., Akangire, G., Sullivan, B., Fairchild, K. & Sampath, V. Continuous vital sign analysis for predicting and preventing neonatal diseases in the twenty-first century: big data to the forefront. Pediatr. Res. 87, 210–220 (2020).
Author information
Authors and Affiliations
Contributions
Both authors made substantial contributions to this manuscript including review of the literature, drafting the paper, and revising it critically for important intellectual content. Both authors gave final approval of the submitted manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sullivan, B.A., Fairchild, K.D. Vital signs as physiomarkers of neonatal sepsis. Pediatr Res 91, 273–282 (2022). https://doi.org/10.1038/s41390-021-01709-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01709-x
This article is cited by
-
Urinary tract infections in very premature neonates: the definition dilemma
Journal of Perinatology (2024)
-
Impact of race on heart rate characteristics monitoring in very low birth weight infants
Pediatric Research (2023)
-
Cardiorespiratory signature of neonatal sepsis: development and validation of prediction models in 3 NICUs
Pediatric Research (2023)
-
Artificial and human intelligence for early identification of neonatal sepsis
Pediatric Research (2023)
-
Global, regional, and national burden of neonatal sepsis and other neonatal infections, 1990–2019: findings from the Global Burden of Disease Study 2019
European Journal of Pediatrics (2023)