Abstract
Background
Low levels of insulin-like growth factor-1 (IGF-1) protein in preterm human infants are associated with bronchopulmonary dysplasia (BPD). We used our preterm lamb model of BPD to determine (1) dosage of recombinant human (rh) IGF-1 bound to binding protein-3 (IGFBP-3) to reach infant physiologic plasma levels; and (2) whether repletion of plasma IGF-1 improves pulmonary and cardiovascular outcomes.
Methods
Group 1: normal, unventilated lambs from 128 days gestation through postnatal age 5 months defined normal plasma levels of IGF-1. Group 2: continuous infusion of rhIGF-1/rhIGFBP-3 (0.5, 1.5, or 4.5 mg/kg/day; n = 2) for 3 days in mechanically ventilated (MV) preterm lambs determined that 1.5 mg/kg/day dosage attained physiologic plasma IGF-1 concentration of ~125 ng/mL, which was infused in four more MV preterm lambs.
Results
Group 1: plasma IGF-1 protein increased from ~75 ng/mL at 128 days gestation to ~220 ng/L at 5 months. Group 2: pilot study of the optimal dosage (1.5 mg/kg/day rhIGF-1/rhIGFBP-3) in six MV preterm lambs significantly improved some pulmonary and cardiovascular outcomes (p < 0.1) compared to six MV preterm controls. RhIGF-1/rhIGFBP-3 was not toxic to the liver, kidneys, or lungs.
Conclusions
Three days of continuous iv infusion of rhIGF-1/rhIGFBP-3 at 1.5 mg/kg/day improved some pulmonary and cardiovascular outcomes without toxicity.
Impact
-
Preterm birth is associated with rapid decreases in serum or plasma IGF-1 protein level. This decline adversely impacts the growth and development of the lung and cardiovascular system. For this pilot study, continuous infusion of optimal dosage of rhIGF-1/rhIGFBP-3 (1.5 mg/kg/day) to maintain physiologic plasma IGF-1 level of ~125 ng/mL during mechanical ventilation for 3 days statistically improved some structural and biochemical outcomes related to the alveolar formation that would favor improved gas exchange compared to vehicle-control. We conclude that 3 days of continuous iv infusion of rhIGF-1/rhIGFBP-3 improved some physiological, morphological, and biochemical outcomes, without toxicity, in mechanically ventilated preterm lambs.
Similar content being viewed by others
Introduction
About 10,000–15,000 preterm human infants develop bronchopulmonary dysplasia (BPD) in the United States annually.1,2 The incidence of BPD over the past decades has not changed despite improvements in care. BPD develops in preterm human infants who require respiratory support at birth because their lungs are too immature structurally and functionally for efficient respiratory gas exchange. Antenatal factors, such as chorioamnionitis, preeclampsia, or intrauterine growth restriction, and postnatal factors, such as mechanical ventilation (MV), hyperoxia, recurring infection/inflammation, and inadequate nutrition, contribute to the evolution of BPD. While molecular mechanisms remain incompletely identified and understood, disruption of growth factors that are critical for the appropriate development of alveoli and alveolar capillaries is implicated in the pathogenesis of BPD.3,4
Insulin-like growth factor-1 (IGF-1) is an important molecular participant in normal lung development and low levels are implicated in the pathogenesis of BPD.5,6,7 Experimental animal models reveal that impaired IGF-1 signaling leads to alveolar simplification in neonates.8 Normally, the fetal plasma level of IGF-1 protein is acquired transplacentally from the mother. Preterm birth is associated with rapid decreases in serum or plasma IGF-1 protein level compared to the concentrations measured in utero at 23–30 weeks postmenstrual age.5,6,7
Treatment with exogenous IGF-1 was studied in a recent clinical trial and in experimental animal models to reduce BPD. A phase II randomized clinical trial assessed treatment with recombinant human (rh) IGF-1 bound to its binding protein-3 (IGFBP-3) in preterm human infants at risk of developing BPD.9 The preterm human infants were continuously infused with the complex for 3–4 weeks. The primary endpoint was the effect on retinopathy of prematurity, which did not improve. Nonetheless, prespecified secondary analysis revealed that the treated preterm human infants who reached the target range of plasma IGF-1 for ≥70% of the treatment time and comprised the evaluable set had a 53% reduction in severe BPD. Advantageous effects included lower incidence of neonatal respiratory distress syndrome and pulmonary hypertension. A recent study using rat pups showed that daily administration of rhIGF-1/rhIGFBP-3 (0.02, 0.2, 2, or 20 mg/kg) for 14 days preserved lung function and structure in a dose-dependent manner in antenatal and postnatal models of BPD.10 Based on these results, we hypothesized that postnatal treatment with rhIGF-1/rhIGFBP-3 will improve pulmonary and cardiovascular outcomes in preterm lambs managed postnatally by MV. Our pilot study’s results show that 3 days of continuous iv infusion of rhIGF-1/rhIGFBP-3 improved some pulmonary and cardiovascular outcomes, without toxicity, in mechanically ventilated preterm lambs.
Methods
Protocols adhered to APS/NIH guidelines for humane use of animals for research and were prospectively approved by the IACUC at the University of Utah Health Sciences Center.
Plasma IGF-1 protein level in normal unventilated lambs during development
We measured plasma levels of IGF-1 protein by endpoint ELISA, using a human IGF-1 ELISA kit (Mediagnost; Reutlinger, Germany), the reagents for which cross-react with IGF-1 from many species, including sheep. Plasma samples were acid-dissociated from binding proteins prior to analysis of free IGF-1. IGF-1 levels were extrapolated from a standard curve derived from recombinant human IGF-1.
Methods for surgical delivery of fetal lambs are reported by our laboratory.11,12,13,14,15,16,17 Time-dated ewes were anesthetized (ketamine; 10 mg/kg, im) and intubated (isoflurane about 2.5%, inhaled).
We determined normal levels of plasma IGF-1 during fetal and postnatal life in unventilated lambs that were not exposed to antenatal steroids. Normal fetal lambs at ~120, ~130, and ~140 days gestation (n = 3–4/gestation age) were delivered to collect blood samples, while the placental circulation was intact, from a cannula placed in a common carotid artery. Normal term lambs also had blood samples collected from a common carotid artery about 24 h after birth, or at 1, 2, or 5 months of postnatal age (n = 3–4/postnatal age).
Verification of downstream signaling by sheep vascular endothelial cells in vitro
We verified in vitro that IGF-1 led to downstream signaling by sheep vascular endothelial cells (ATCC- Manassas, VA). Cells were seeded at 60,000/well in a 96-well tissue culture plate. The next day, cells were washed once and left overnight in serum-free Dulbecco’s Modified Eagle Medium (Invitrogen, Waltham, MA). After serum starvation, the cells were treated with 0, 10, 50, or 100 ng/mL rhIGF-1 (R&D Systems, Minneapolis, MN) or bovine serum albumin (Fisher Scientific, Waltham, MA) for 5, 10, or 30 min. Cells were washed and then lysed on ice for 30 min in 100 μL lysis buffer (10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 0.5% Triton-x 100), with halt protease and phosphatase inhibitor cocktail (Fisher Scientific). Cell lysates were analyzed for pIGF-1R, according to the manufacturer’s instructions, using AlphaLISA SureFire Ultra kit (catalog #ALSU-PLGFR-A500; PerkinElmer, Downers Grove, IL). Briefly, 10 µL of cell lysate and 5 µL acceptor mix were added to a PerkinElmer white half area plate and incubated for 1 h in the dark at room temperature. Five µL of donor mix was added and incubated in the dark for 1 h at room temperature. The plate was read on an Envision instrument.
Effect of continuous infusion of rhIGF-1/rhIGFBP-3 or vehicle (control) in preterm lambs during invasive mechanical ventilation (MV) for 3 days
A pilot dosage-finding pharmacokinetic study of continuously infused rhIGF-1/rhIGFBP-3 (Mecasermin rinfabate) was done to define the dose of rhIGF-1/rhIGFBP-3 that produced blood levels in the upper bounds of the normal fetal range (112 ± 74 ng/mL), after which the optimized dosage was used to compare outcomes between rhIGF-1/rhIGFBP-3-treated and vehicle-treated control preterm lambs during MV for 3 days.
Methods for delivering preterm lambs are reported by our laboratory.11,12,13,14,15,16 Briefly, time-dated pregnant ewes (singletons or twins) were studied at ~131 days of gestation (saccular stage of lung development). The pregnant ewes were given an intramuscular injection of dexamethasone phosphate (6 mg; Vedco, Inc., St. Joseph, MO) at ~48 h, and ~24 h before Cesarean-section delivery. At delivery, we intubated the fetal lambs with a cuffed endotracheal tube (3.5–4.0 French), through which 10 mL of lung liquid were aspirated and replaced with Infasurf® (3 mL/kg; ONY Biotech, Amherst, NY).
Preterm lambs were resuscitated through the endotracheal tube, using a programmed resuscitation box.18 The lambs were weighed, placed prone on a veterinary sling atop a radiantly heated NICU bed, and connected to a Dräger ventilator (model VN500, Lubeck, Germany). Sedation was the same for all ventilated preterm lambs (pentobarbital as needed and buprenorphine every 6 h).18 Lambs were supported with synchronized intermittent mandatory ventilation that was pressure-controlled, with warmed and humidified gas. FiO2 was adjusted to attain target hemoglobin oxygen saturation of 90–94% (PaO2 60–90 mmHg) by pulse oximetry (Model SurgiVet V9200IBP/Temp, Smith Medical ASD, Inc., St. Paul, MN). Peak inspiratory pressure was adjusted to attain a target PaCO2 between 45 and 60 mmHg, resulting in pH between 7.25 and 7.35. Target expiratory tidal volume, measured by the ventilator, was 5–7 mL/kg. We calculated oxygenation index (OI) [(Paw × FiO2)/PaO2], P/F (PaO2/FiO2) ratio, and Alveolar-arterial (A-a) gradient [((FiO2/100) × (640–47)) – (PaCO2/0.8)-PaO2]. Salt Lake City is at about 5000 ft elevation so barometric pressure is about 640 mmHg.
Orogastric feeding of ewe’s colostrum (Kid & Lamb Colostrum Replacement, Land O Lakes, Arden Hills, MN) was started at ~3 h of postnatal life (3 mL) and the volume was gradually increased as tolerated, with a target of ~60 kcal/kg/day. Parenteral dextrose was infused to maintain plasma glucose between 60 and 90 mg/dL. Arterial blood samples were collected every 24 h to measure plasma levels of IGF-1 protein, as well as indicators of liver and kidney injury (analyzed at Associated Regional and University Pathologists (ARUP) Laboratories, Salt Lake City).
We used continuous infusion of rhIGF-1/rhIGFBP-3 because we determined that IGF-1 has a half-life in plasma of ~2 h in lambs. The tested dosages were 0.5, 1.5, or 4.5 mg/kg/day. Supplementary Fig. 1 shows plasma IGF-1 levels for the dose-optimization studies (2 preterm lambs per dosage and 2 matched preterm lambs received vehicle (saline) for each dosage; n = 12). The average plasma level of IGF-1 protein at day of life 3 (72 h) was 62 ng/mL for 0.5 mg/kg/day dosage (range 60–64 ng/mL), 168 ng/mL for 1.5 mg/kg/day dosage (range 125–220 ng/mL), and 330 ng/mL for 4.5 mg/kg/day dosage (range 220–400 ng/mL). At day of life 3, the 1.5 mg/kg/day dosage regimen was associated with apparently higher oxygen saturation by pulse oximetry and PaO2 for comparable FiO2, and higher mean aortic blood pressure, as well as lower peak inspiratory pressure and PaCO2, relative to the other two dosage regimens and vehicle controls. Based on these results, the optimal dosage was identified as 1.5 mg/kg/day. We used the optimized dosage regimen in four more preterm lambs (n = 6). Four more ventilated preterm lambs were treated with vehicle (n = 6). The additional preterm lambs were assigned to rhIGF-1/rhIGFBP-3 treatment or vehicle treatment by a blinded selection before surgical delivery to minimize bias.
Terminal tissue collection of the lung from preterm lambs
At the end of MV for 3 days, blood samples were collected before the preterm lambs were given heparin (1000 U, intravenously) followed by 5 mg/kg of pentobarbital.16,18 Lambs were subsequently given 60 mg/kg pentobarbital sodium solution intravenously (Beuthanasia solution, Ovation Pharmaceuticals, Inc., Deerfield, IL). The chest was opened, the trachea was ligated at end-inspiration (to minimize atelectasis), and the lungs and heart were removed. The whole left lung was insufflated with 10% buffered neutral formalin at a static pressure of 25 cmH2O. Fixed-lung displacement volume was measured by suspension in formalin before the lung was stored in fixative (4 °C, 24 h). Paraffin-embedded tissue blocks were prepared for histology and quantitative histology, including quantitative immunohistochemistry to assess structural indices of alveolar formation and alveolar capillary growth.12,16,18,19 We used immunostained sections of lung tissue to quantify indices of alveolar capillary growth (PECAM-1 immunostain) and blue counterstain to identify epithelial cells. The right caudal lobe of the lung was used for molecular analyses (snap-frozen in liquid nitrogen and stored at −80 °C).12,16,18,19 We used systematic, uniform, and random, protocols for an unbiased sampling of lung tissue.20
Data analysis
For this pilot study, we prospectively used α = 0.1 (90%) to identify potential advantageous outcomes at the end of 3 days of treatment with the optimized dosage of rhIGF-1/rhIGFBP-3. Results are summarized as mean ± standard deviation (SD) or mean (interquartile range, IQR), as shown in the tables and figures. Statistical analyses were done using GraphPad (Prism, v9). We used two-way ANOVA (treatment and time), followed by post hoc Holm–Šídák’s multiple comparisons test for physiological results. We used a one-tailed parametric t-test for morphological outcomes and one-tailed non-parametric tests for immunoblot results.
Results
Plasma IGF-1 protein level in normal unventilated lambs during development
Plasma IGF-1 protein level increased from ~75 ng/mL in normal unventilated fetal lambs to ~220 ng/mL through 5 months postnatal age in normal unventilated term lambs (Fig. 1a).
a During normal development from fetal lambs (about 120 days gestation) to adolescent lambs (about 150 days of age), IGF-1 protein level progressively increased (Pearson r = 0.540; p = 0.004). b Group 2 plasma level of IGF-1 protein in preterm lambs managed by invasive mechanical ventilation for 3 days. Continuous iv infusion of rhIGF-1/rhIGFBP-3 (1.5 mg/kg/day; black squares) attained the target plasma level of about 125 ng/mL for the last 48 h of the 72 h study, whereas for vehicle-control preterm lambs (white circles), plasma IGF-1 protein level significantly decreased to about 30 ng/mL for the last 48 h of the 72 h study period. Symbols for statistical differences: single line = significantly lower level from 12 through 72 h for the vehicle-control preterm lambs compared to this group’s “predose” level; * = significantly higher level from 12 through 72 h for the rhIGF-1/rhIGFBP-3-treated preterm lambs compared to this group’s “predose” level; double lines = significantly greater for the rhIGF-1/rhIGFBP-3-treated preterm lambs compared to the matched hour’s level for the vehicle-control preterm lambs. Statistical analyses for panel b were by two-way ANOVA and Holm–Šídák’s multiple comparisons test, with α = 0.05 (95%).
Effect of continuous infusion of rhIGF-1/rhIGFBP-3 or vehicle (control) in preterm lambs during invasive mechanical ventilation (MV) for 3 days
Demographic characteristics of this randomized, placebo-controlled study are summarized in Table 1. Gestational age, birth weight, and ending weight were not statistically different between the two sets of ventilated preterm lambs. Nonetheless, at operative delivery, the rhIGF-1/rhIGFBP-3-treated preterm lambs’ gestation age was 1 day younger and delivery weight was about 0.5 kg lower than for the vehicle-control preterm lambs. Female:male distribution was not equal between the rhIGF-1/rhIGFBP-3-treated and vehicle-control-treated preterm lambs. This was not possible because treated or vehicle-control group assignment occurred before operative delivery of fetuses.
Plasma IGF-1 protein level in fetal lambs, while the placental circulation was intact before operative delivery, was the same for both sets of ventilated preterm lambs (Fig. 1b; “predose” level was ~100 ng/mL; not statistically different). Subsequently, plasma IGF-1 protein level diverged between the rhIGF-1/rhIGFBP-3-treated and vehicle-control-treated preterm lambs over the 3-day study period. For preterm lambs treated with 1.5 mg/kg/day rhIGF-1/rhIGFBP-3, plasma IGF-1 protein level doubled (220 ± 60 ng/mL) at 12 h of continuous infusion compared to this set’s pretreatment baseline level (103 ± 63 ng/mL; p < 0.05). The IGF-1 protein level plateaued at about 140 ng/mL for the last 24 h of the 3-day study period (p < 0.05 compared to this set’s pretreatment baseline level). For the vehicle-treated preterm lambs, by comparison, plasma IGF-1 protein level significantly decreased from the set’s baseline level (91 ± 40 ng/mL) to a nadir of about 30 ng/mL for the last 48 h of the 3-day study period (36 ± 25 ng/mL at 60 and 72 h; p < 0.05).
Human IGF-1 led to phosphorylation of IGF-1R in sheep endothelial cells in vitro, as shown in Fig. 2. The response was concentration-dependent (Fig. 2a). Only 50 ng/mL and 100 ng/mL rhIGF-1 treatments led to levels above background at all timepoints tested (Fig. 2b). Control (bovine serum albumin) treatment did not lead to signal elevation across all timepoints and dose ranges.
Physiological parameters for respiratory gas exchange are presented in Fig. 3. Results are shown for 12 h epochs of postnatal age during the 3 days of MV. Targets were SaO2 range 90–94% (PaO2 range 60–90 mmHg) for oxygenation and PaCO2 range 45–60 mmHg for ventilation. Although numerical results favored rhIGF-1/rhIGFBP-3 treatment, no statistical differences were detected between the rhIGF-1/rhIGFBP-3-treated versus vehicle-control preterm lambs for the applied FiO2 or PIP to sustain the oxygenation and ventilation targets, respectively (Fig. 3a–d). OI and A-a gradient were significantly improved in rhIGF-1/rhIFGBP-3 treated lambs (Table 2). Plasma pH and bicarbonate also were not different between the rhIGF-1/rhIGFBP-3-treated and vehicle-control preterm lambs over the 3-day study period (Fig. 3e, f, respectively). No differences were detected in the amount of pentobarbital (mg/kg/12 h, iv) or buprenorphine (mcg/kg/12 h, iv) between the rhIGF-1/rhIGFBP-3-treated preterm lambs compared to the vehicle-control preterm lambs (data not shown).
Continuous iv infusion of rhIGF-1/rhIGFBP-3 (1.5 mg/kg/day; blacks squares) led to somewhat better respiratory gas exchange (a–f), as well as systemic hemodynamic and heart rate outcomes (g–j) relative to vehicle-control preterm lambs (open circles); however, no statistically significant differences (p > 0.1) were detected.
The systemic hemodynamic parameters shown in Fig. 3 are numerically better for the rhIGF-1/rhIGFBP-3-treated preterm lambs; however, results were not statistically different from the vehicle-control preterm lambs (Fig. 3g–j, respectively). Consistent with other cardiovascular parameters, none of the six rhIGF-1/rhIGFBP-3-treated preterm lambs required dopamine to maintain mean aortic pressure >35 mmHg (Table 3), whereas two of the six vehicle-control preterm lambs required dopamine infusion (one lamb required 7 mcg/kg/min during hours of life 33–72; another lamb required 6 mcg/kg/min during hours of life 6–48, continuing with 4 mcg/kg/min through 72 h).
Fluid balance parameters are summarized in Supplementary Table 1. Average results are reported for 12 h epochs. No statistical differences were detected for intravenous infusion of saline or dextrose, total fluid intake, enteral milk intake, or urine output between the rhIGF-1/rhIGFBP-3-treated versus vehicle-control preterm lambs.
Liver function and kidney function test values are summarized in Tables 3 and 4, respectively. Plasma samples were taken while fetal lambs had their placental circulation intact (“pre” in Tables 3 and 4), and at “24 h” and “72 h”. Liver function was assessed by measurement of plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AP), total bilirubin, and direct bilirubin. Indicators of kidney function were urine output, creatinine, blood urea nitrogen, lactate, and urine microalbumin. No differences (p > 0.1) were detected in liver function or kidney function indices between the rhIGF-1/rhIGFBP-3-treated versus the vehicle-control preterm lambs. The levels were within reference limits for fetal lambs, adult sheep, and adult humans (Tables 3 and 4).
Histological examples of alveolar architecture are shown in Fig. 4. Alveolar architecture appears similar between the rhIGF-1/rhIGFBP-3-treated and vehicle-control preterm lambs at the end of 3 days of MV. As expected, after 3 days of MV, the vehicle-control preterm lambs had terminal respiratory units (TRU) that were distended and simplified. That is, the parenchyma was thick and cellular, and few buds of alveolar secondary septa were evident. TRU architecture appeared somewhat more developed, with short buds of secondary septa being more evident in the lung of the rhIGF-1/rhIGFBP-3-treated preterm lambs. Quantitative histological indices of alveolar formation revealed no statistical differences between the rhIGF-1/rhIGFBP-3-treated and vehicle-control preterm lambs for radial alveolar count, secondary septal volume density, or distal airspace wall thickness (Fig. 4e–g).
Continuous iv infusion of rhIGF-1/rhIGFBP-3 (1.5 mg/kg/day) led to somewhat better appearing terminal respiratory units (TRU; b) and secondary septa (arrow in d) relative to vehicle-control (a, c). Panels a, b are the same magnification; see scale bar. Panels c, d are the same magnification; see scale bar. Distal airspace walls (arrowhead in c, d) appear to have a similar thickness between the two groups. Quantitative histology revealed no statistical differences (p > 0.1) for radial alveolar count, secondary septal volume density, or distal airspace wall thickness between the rhIGF-1/rhIGFBP-3-treated preterm lambs and vehicle-control preterm lambs (e–g).
Histological examples of alveolar capillary endothelial cell identification by immunohistochemistry are shown in Fig. 5. Stereological assessment of surface density detected statistically significantly larger surface density for capillary endothelial cells and airspace epithelial cells for the rhIGF-1/rhIGFBP-3-group compared to the vehicle-control preterm lambs (p < 0.1).
Immunohistochemistry was used to label capillary endothelial cells (brown color in a, b; the panels are the same magnification; see scale bar). Quantitative histology (c, d) showed that continuous iv infusion of rhIGF-1/rhIGFBP-3 (1.5 mg/kg/day; black squares) led to significantly greater capillary surface density (p < 0.1) and epithelial surface density (p < 0.1) compared to vehicle-control (white circles).
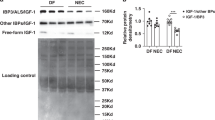
Semi-quantitative protein abundance in lung parenchyma is shown in Fig. 6. Statistical difference was detected for cleaved caspase 3, for which the relative protein abundance was significantly greater for the rhIGF-1/rhIGFBP-3-treated preterm lambs compared to the vehicle-control preterm lambs (p < 0.1). Otherwise, no statistical differences were detected for protein abundance of proliferating cell nuclear antigen (PCNA) or fetal liver kinase-1 (Flk-1) between the two groups.
Continuous iv infusion of rhIGF-1/rhIGFBP-3 (1.5 mg/kg/day; black squares) led to significantly greater abundance of cleaved caspase 3 (b; p < 0.1) compared to the vehicle-control (white circles). No statistical differences were detected for normalized protein abundance of proliferating cell nuclear antigen (PCNA; a) or fetal liver kinase-1 (Flk-1 (VEGF-R2); c).
Discussion
The current study was designed to model the situation in preterm human infants. The study design took two steps. The first step was to establish that the IGF-1 system in preterm lambs is a phenocopy of that in preterm human infants, with a rapid fall in the blood level of IGF-1 after preterm delivery, a period when organ maturation is driven in substantial part by IGF-1. Our results show that during normal development, IGF-1 protein level in plasma increased from ~100 ng/mL in unventilated fetuses at about 128 days gestation (equivalent to 28 weeks gestation in humans) to about 220 ng/mL in unventilated lambs at 5 months postnatal age (equivalent to about 6 years in humans).21 The normal blood IGF-1 level in the human fetus between 23 and 28 weeks gestation is 28–109 ng/mL.22,23
The second step was to establish an optimized dose of IGF-1 for preterm lambs that provided a physiologic replacement. To attain this step, we first found, in vehicle-control preterm lambs, that IGF-1 protein level decreased significantly during 3 days of MV, like in preterm human infants. We subsequently established an optimal dosage of rhIGF-1/rhIGFBP-3 (1.5 mg/kg/day) to maintain the physiologic plasma IGF-1 level. The endpoint was a dose of rhIGF-1/rhIGFBP-3 that produced blood levels in the upper bounds of the normal fetal range (mean ± 1 SD). For the initial 12 preterm lambs that we studied, the “pre” level (intact umbilical cord) of IGF-1 was 112 ± 74 ng/mL. Therefore, the physiological replacement target range was 112–186 ng/mL. We used that dosage to evaluate the pulmonary and cardiovascular physiological, and lung structural and biochemical effects of 3 days’ continuous infusion of rhIGF-1/rhIGFBP-3 in mechanically ventilated preterm lambs (n = 6). We chose 3 days of MV because our previous studies showed that lung function and structure in preterm lambs are different during invasive versus noninvasive respiratory support.12,19 Continuous infusion of rhIGF-1/rhIGFBP-3 during MV for 3 days statistically improved some pulmonary and cardiovascular outcomes compared to vehicle-control preterm lambs. Systemic hypotension did not occur in rhIGF-1/rhIGFBP-3-treated preterm lambs (0/6), whereas 2/6 vehicle-control preterm lambs required dopamine to maintain physiologic systemic perfusion pressure. Prior studies in our laboratory show that preterm lambs that are managed by MV develop systemic hypotension that requires dopamine treatment. Also, rhIGF-1/rhIGFBP-3-treated preterm lambs maintained their weight, whereas vehicle-control preterm lambs lost weight from day of life 1 through day of life 3 (p < 0.1). Furthermore, some structural and biochemical outcomes related to the alveolar formation that would favor improved gas exchange were statistically better in the IGF-1/IGFBP-3-treated preterm lambs compared to vehicle-control preterm lambs at the end of the 3-day study. Another result is that rhIGF-1/rhIGFBP-3 infusion did not adversely affect the liver and kidneys of the preterm lambs. We conclude from this pilot study that 3 days of continuous iv infusion for physiological replacement of rhIGF-1/rhIGFBP-3 improved some physiological, morphological, and biochemical outcomes, without toxicity, in mechanically ventilated preterm lambs.
Angiogenesis is an important process for appropriate alveolar formation in the lung. Angiogenesis is diminished in preterm human infants with BPD and in animal models of BPD, contributing to alveolar simplification.11,24,25 A relevant underlying molecular impact of IGF-1 treatment is the increased growth of vascular endothelial cells.26,27,28,29 A recent study in rat pups showed that daily intraperitoneal treatment with 0.02–20 mg/kg of rhIGF-1/rhIGFBP-3 for 14 days preserved lung structure in a dose-dependent manner and prevented right ventricular hypertrophy in antenatal and postnatal models of BPD.10 That study showed that exogenous IGF-1 enhanced pulmonary vascular endothelial cell growth and tube formation. Therefore, we focused on endothelial cells. Our results show that stereological indices of capillary endothelial cell and airspace epithelial cell surface density were statistically larger in the lungs of the rhIGF-1/rhIGFBP-3-treated group compared to the vehicle-control preterm lambs (p < 0.1). Therefore, therapeutic strategies such as exogenous IGF-1 treatment may provide a novel approach toward the prevention of BPD following early diagnosis of high-risk preterm infants.
This study’s immunoblot results provide some insight into the impact of IGF-1 on lung outcomes. The statistically greater abundance of cleaved caspase 3 protein detected in parenchymal tissue of the lung of the rhIGF-1/rhIGFBP-3-treated preterm lambs is consistent with the results of our previous studies.12,19 Those studies showed that noninvasive respiratory support led to greater protein abundance of cleaved caspase 3 that localized to mesenchymal cells in the thinner segments of the saccular walls compared to invasive MV for 3 or 21 days. In this regard, greater abundance of cleaved caspase 3 in the lung of the rhIGF-1/rhIGFBP-3-treated preterm lambs is consistent with improving structural thinning of saccular walls. On the other hand, the current study did not detect a significant decrease in PCNA protein abundance, which is not consistent with the results of our previous studies.12,19 Nonetheless, PCNA relative protein abundance was numerically lower in the rhIGF-1/rhIGFBP-3-treated preterm lambs, which is at least consistent with our previous findings. These results will require further investigation because IGF-1 signaling, while typically protective against apoptosis,30,31 also is proapoptotic.32,33
An important consideration for potential treatments for BPD is adverse effects. An important observation in our study is that neither liver function nor kidney function indices were adversely affected during 3 days of continuous intravenous infusion of rhIGF-1/rhIGFBP-3. IGF-1 is eliminated by the kidneys.34,35 Free IGF-1, particularly when administered without binding protein-3, can activate the insulin receptor and cause hypoglycemia. Mild elevations of hepatic enzymes and renal enlargement without functional impairment have been observed in IGF-I clinical trials in indications other than prematurity.36 These safety signals were not observed in trials of rhIGF-1/rhIGFBP-3 in preterm infants who received an infusion of ≤250 µg/kg/day continuously for up to 45 days.9
Our study’s design has strengths. One strength is using a large-animal model that emulates preterm birth and prolonged respiratory management, without hyperoxia, in a neonatal intensive care setting. Another strength is the breadth of assessments, from feeding tolerance and growth, respiratory gas exchange, cardiovascular physiology, and structural and biochemical indices relevant to alveolar formation to indices of liver and kidney function.
Our study has limitations, too. A limitation of the present study is the preterm model is a non-lethal model that uses fetal lambs delivered at about 85% of gestation (saccular stage of lung development; equivalent to about 28 weeks gestation in humans). Also, the duration of MV and exposure to rhIGF-1/rhIGFBP-3 was short, lasting only 3 days for this pilot study. Nonetheless, the sheep endothelial cell experiments in vitro showed that downstream signaling was triggered by rhIGF-1. Three-day duration of continuous infusion of rhIGF-1/rhIGFBP-3 may have been insufficient for IGF-1’s morphogenic effects. Indeed, a recent study of postnatal rhIGF-1/rhIGFBP-3 treatment of rat pups after prolonged hyperoxia (90% O2) found that limiting rhIGF-1/rhIGFBP-3 treatment to 3 days did not lead to beneficial effects.10 Two weeks of treatment was efficacious.10 Another limitation is that antenatal stress (e.g., chorioamnionitis or preeclampsia) was not part of our model. Nonetheless, our study provides new insights that provide preliminary support for the beneficial effects of continuous iv infusion of rhIGF-1/rhIGFBP-3 into mechanically ventilated preterm neonates.
In summary, we report that continuous infusion of rhIGF-1/rhIGFBP-3 increased plasma IGF-1 protein level in preterm lambs managed by MV for 3 days whereas that level progressively decreased in vehicle-control preterm lambs. Replenishment of rhIGF-1/rhIGFBP-3 improved some lung functional and structural phenotypic characteristics. Thus, our pilot study provides new mechanistic insight into potential effective treatment for preterm human infants at-risk for developing BPD. Our ongoing studies are testing longer exposure of preterm lambs to rhIGF-1/rhIGFBP-3 and using a larger sample size.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Northway, W. H. Jr., Rosan, R. C. & Porter, D. Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Eng. J. Med. 276, 357–368 (1967).
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051 (2015).
Voller, S. B. et al. Cord blood biomarkers of vascular endothelial growth (VEGF and sFlt-1) and postnatal growth: a preterm birth cohort study. Early Hum. Dev. 90, 195–200 (2014).
Olmos, A. et al. Associations between insulin-like growth factor I, vascular endothelial growth factor and its soluble receptor 1 in umbilical serum and endothelial cells obtained from normotensive and preeclamptic pregnancies. Growth Factors 31, 123–129 (2013).
Hellström, A. et al. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 105, 576–586 (2016).
De Paepe, M. E. et al. Growth of pulmonary microvasculature in ventilated preterm infants. Am. J. Respir. Crit. Care Med. 173, 204–211 (2006).
Lassarre, C. et al. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr. Res. 29, 219–225 (1991).
Li, J. et al. The IGF-I/IGF-R1 pathway regulates postnatal lung growth and is a nonspecific regulator of alveologenesis in the neonatal rat. Am. J. Physiol. Lung Cell Mol. Physiol. 304, L626–L637 (2013).
Ley, D. et al. rhIGF-1/rhIGFBP-3 in preterm infants: a phase 2 randomized controlled trial. J. Pediatr. 206, 56–65.e58 (2019).
Seedorf G., et al. rhIGF-1/BP3 preserves lung growth and prevents pulmonary hypertension in experimental BPD. Am. J. Respir. Crit. Care Med. 201, 1120–1134 (2020).
Albertine, K. H. et al. Chronic lung injury in preterm lambs: disordered respiratory tract development. Am. J. Respir. Crit. Care Med. 159, 945–958 (1999).
Reyburn, B. et al. Nasal ventilation alters mesenchymal cell turnover and improves alveolarization in preterm lambs. Am. J. Respir. Crit. Care Med. 178, 407–418 (2008).
Albertine, K. H. et al. Chronic lung disease in preterm lambs: effect of daily vitamin A treatment on alveolarization. Am. J. Physiol. Lung Cell Mol. Physiol. 299, L59–L72 (2010).
Rehan, V. K. et al. Mechanism of reduced lung injury by high-frequency nasal ventilation in a preterm lamb model of neonatal chronic lung disease. Pediatr. Res. 70, 462–466 (2011).
Joss-Moore L. A., et al. Alveolar formation is dysregulated by restricted nutrition but not excess sedation in preterm lambs managed by non-invasive support. Pediatr. Res. 80, 719–728 (2016).
Dahl, M. J. et al. Former-preterm lambs have persistent alveolar simplification at 2 and 5 months corrected postnatal age. Am. J. Physiol. Lung Cell Mol. Physiol. 315, L816–L833 (2018).
Liu, M. et al. Transforming growth factor-induced protein promotes NF-kappaB-mediated angiogenesis during postnatal lung development. Am. J. Respir. Cell Mol. Biol. 64, 318–330 (2021).
Dahl, M. J. et al. Early extubation to noninvasive respiratory support of former preterm lambs improves long-term respiratory outcomes. Am. J. Physiol. Lung Cell Mol. Physiol. 321, L248–L262 (2021).
Null, D. M. et al. High-frequency nasal ventilation for 21 d maintains gas exchange with lower respiratory pressures and promotes alveolarization in preterm lambs. Pediatr. Res. 75, 507–516 (2014).
Hsia, C. C., Hyde, D. M., Ochs, M. & Weibel, E. R. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am. J. Respir. Crit. Care Med. 181, 394–418 (2010).
Unsworth, W. P., Taylor, J. A. & Robinson, J. E. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biol. Reprod. 72, 619–627 (2005).
Löfqvist, C. et al. Low postnatal serum IGF-I levels are associated with bronchopulmonary dysplasia (BPD). Acta Paediatr. 101, 1211–1216 (2012).
Chung, J. K. et al. Development and verification of a pharmacokinetic model to optimize physiologic replacement of rhIGF-1/rhIGFBP-3 in preterm infants. Pediatr. Res. 81, 504–510 (2017).
Jakkula, M. et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L600–L607 (2000).
Le Cras, T. D. et al. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am. J. Physiol. Lung Cell Mol. Physiol. 283, L555–L562. (2002).
Hellström, A. et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc. Natl Acad. Sci. USA 98, 5804–5808 (2001).
Epaud, R. et al. Knockout of insulin-like growth factor-1 receptor impairs distal lung morphogenesis. PLoS One 7, e48071 (2012).
Bach, L. A. Endothelial cells and the IGF system. J. Mol. Endocrinol. 54, R1–R13 (2015).
Jacobo, S. M. & Kazlauskas, A. Insulin-like growth factor 1 (IGF-1) stabilizes nascent blood vessels. J. Biol. Chem. 290, 6349–6360 (2015).
Zhang, M. et al. Insulin-like growth factor 1/insulin-like growth factor 1 receptor signaling protects against cell apoptosis through the PI3K/AKT pathway in glioblastoma cells. Exp. Ther. Med. 16, 1477–1482 (2018).
Liu, Q. et al. Insulin-like growth factor 1 receptor-mediated cell survival in hypoxia depends on the promotion of autophagy via suppression of the PI3K/Akt/mTOR signaling pathway. Mol. Med. Rep. 15, 2136–2142 (2017).
Remacle-Bonnet, M. et al. Membrane rafts segregate pro- from anti-apoptotic insulin-like growth factor-I receptor signaling in colon carcinoma cells stimulated by members of the tumor necrosis factor superfamily. Am. J. Pathol. 167, 761–773 (2005).
Kooijman, R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev. 17, 305–323 (2006).
Kimura, T. et al. Disposition of recombinant human insulin-like growth factor-I in normal and hypophysectomized rats. Biol. Pharm. Bull. 17, 310–315 (1994).
Mizuno, N. et al. Kinetic analysis of the disposition of insulin-like growth factor 1 in healthy volunteers. Pharm. Res. 18, 1203–1209 (2001).
FDA. Increlex Insert. Reference ID: 3517143. https://www.accessdata.fda.gov 7–8 (2014).
Acknowledgements
We thank the dozens of undergraduate students and medical students who learned and provided neonatal intensive care to the preterm lambs. We also thank Angela Presson, PhD, MS, at the University of Utah for guidance for the study’s pilot design and statistical analysis, as well as Jennifer Bosco, PhD, and Bettina Stack-Logue, PhD, at Takeda Pharmaceutical Company who provided in vitro pharmacology support to characterize the effect of rhIGF-1 on sheep IGF-1 receptors.
Funding
Supported by research grant awards from Takeda (formerly Shire) Pharmaceuticals and NIH R01 HL110002 (K.H.A.) and T35 HL007744 (supported C.M.), and the Division of Neonatology, Department of Pediatrics, University of Utah.
Author information
Authors and Affiliations
Contributions
K.H.A.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. M.J.D.: Substantial contributions to acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. A.R.: Substantial contributions to acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. E.D.: Substantial contributions to acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. A.N.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. S.B.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. C.M.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. Z.W.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. H.Y.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. B.Y.: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. D.M.N.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. D.K.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. J.-K.C.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. Z.Z: Substantial contributions to acquisition of data, or analysis and interpretation of data; and final approval of the version to be published. N.B.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. G.C.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. R.W.: Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
A competing interest is identified because the study was funded by an independent research grant award from Takeda Pharmaceuticals to the University of Utah for this study to be done in Dr Albertine’s lamb intensive care unit.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albertine, K.H., Dahl, M.J., Rebentisch, A. et al. Pilot dose-ranging of rhIGF-1/rhIGFBP-3 in a preterm lamb model of evolving bronchopulmonary dysplasia. Pediatr Res 93, 1528–1538 (2023). https://doi.org/10.1038/s41390-022-02272-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02272-9