Abstract
White matter (WM) injury is the most common type of brain injury in preterm infants and is associated with impaired neurodevelopmental outcome (NDO). Currently, there are no treatments for WM injury, but optimal nutrition during early preterm life may support WM development. The main aim of this scoping review was to assess the influence of early postnatal nutrition on WM development in preterm infants. Searches were performed in PubMed, EMBASE, and COCHRANE on September 2022. Inclusion criteria were assessment of preterm infants, nutritional intake before 1 month corrected age, and WM outcome. Methods were congruent with the PRISMA-ScR checklist. Thirty-two articles were included. Negative associations were found between longer parenteral feeding duration and WM development, although likely confounded by illness. Positive associations between macronutrient, energy, and human milk intake and WM development were common, especially when fed enterally. Results on fatty acid and glutamine supplementation remained inconclusive. Significant associations were most often detected at the microstructural level using diffusion magnetic resonance imaging. Optimizing postnatal nutrition can positively influence WM development and subsequent NDO in preterm infants, but more controlled intervention studies using quantitative neuroimaging are needed.
Impact
-
White matter brain injury is common in preterm infants and associated with impaired neurodevelopmental outcome.
-
Optimizing postnatal nutrition can positively influence white matter development and subsequent neurodevelopmental outcome in preterm infants.
-
More studies are needed, using quantitative neuroimaging techniques and interventional designs controlling for confounders, to define optimal nutritional intakes in preterm infants.
Similar content being viewed by others
Introduction
Preterm infants are born during a critical period of brain development, making them susceptible to brain injury1 and subsequent neurodevelopmental impairment.2 During the third trimester of pregnancy, brain volume increases, the cortex becomes folded, and underneath a complex white matter network is formed.3 Within the white matter, pre-oligodendrocytes differentiate into mature oligodendrocytes that myelinate the first axons around 30 weeks of gestational age, and continue to increase myelination during the first 5 years of life.4 However, these pre-oligodendrocytes are extremely vulnerable to inflammation and oxygen fluctuations, which often occur in preterm infants. As a result, white matter injury is the most common type of brain injury in extremely preterm infants,5,6 which is associated with impaired neurodevelopmental outcome (NDO).6,7 The most severe types of white matter injury are cystic periventricular leukomalacia (PVL) and periventricular hemorrhagic infarction.6 The incidence of PVL has declined over the past years due to improved perinatal care, making non-cystic diffuse white matter injury the predominant form of white matter injury in preterm infants.8
Although there are no treatment options available for white matter injury, early postnatal nutrition seems a promising and modifiable candidate to reduce white matter injury,9,10,11,12 thereby improving white matter development. Different nutritional components, including human milk, fatty acids, vitamins, and minerals, could have anti-inflammatory effects by influencing cytokine production.13,14,15,16 Other nutritional components, such as glutamine, probiotics, and prebiotics, dampen the inflammatory response by modulating the microbiome–gut–brain axis.11 Furthermore, certain nutrients, including protein, iron, zinc, selenium, iodine, folate, vitamin A, choline, and certain fatty acids, affect brain development by providing specific substrates, synthesizing, and activating growth factors.17 Hence, early postnatal nutrition could influence white matter development via different pathways.
Several studies have demonstrated a relationship between early nutritional intake and white matter development in preterm infants.18,19,20 However, these studies are heterogeneous in study design, methods, and results. Previous reviews on nutrition and brain development focused primarily on pre-clinical studies,21 term infants,10 NDO,22,23 undernutrition,9 potential underlying mechanisms,11 or overall brain development24 in relation to single nutritional components.25 To our knowledge, no review to date focused primarily on early postnatal nutrition in relation to white matter development in preterm infants. Therefore, the main aim of this scoping review is to answer the following question: What is the influence of early postnatal nutrition on white matter development in preterm infants? Secondary aims were to (1) identify factors that determine the efficacy of nutritional intake on white matter development, such as specific nutritional components, timing, and dose, (2) to assess NDO in relation to nutritional intake, and (3) to identify research gaps in order to guide future research.
Methods
The methodology manual as published by the Joanna Briggs Institute for scoping reviews26 was used, which is congruent with the PRISMA checklist extended for scoping reviews (PRISMA-ScR).27 This protocol was pre-registered in the Open Science Framework (http://osf.io/aphuk).
Eligibility criteria
Studies were included if they assessed nutritional intake, in a period between birth and 1 month corrected age, in relation to white matter development or injury, either observational or as part of an intervention, in infants born before 37 weeks of gestation and/or having a birthweight below 1500 g. Studies had to be published in a peer-reviewed journal.
Studies were excluded in case of unavailable full text, double reporting, pre-clinical studies, reviews, conference papers, descriptive case reports, and commentaries. Studies using functional magnetic resonance imaging (MRI) or electrophysiological measures were not included in this review as these are primarily measures of cortical activity. No limitations were set on language and publication date.
Search strategy
The following electronic bibliographic databases were searched: PubMed, EMBASE, and COCHRANE. An initial limited search in PubMed was undertaken to identify search terms in the titles and the abstracts of relevant articles. The search strategies were drafted in collaboration with an experienced librarian (P.W.) and further refined through team discussion. The final search strategy for all databases can be found in Appendix 1. The reference lists of all included articles were screened for additional studies.
Study selection
A first search was performed on March 25, 2021. The records were uploaded into Zotero to remove duplicates (www.zotero.org).28 Title and abstract screening was performed in Rayyan (http://rayyan.qcri.org)29 by two reviewers independently (E.J., P.E.v.B.), following a pilot test on a random sample of 100 records. The search was updated on September 27, 2022. Duplicates between the searches were removed in EndNote X9, with the help of an experienced librarian (M.L.S.G.). Title and abstract screening were again performed by two reviewers independently (E.J., M.F.W.). The full texts of all selected articles were assessed in detail against the in- and exclusion criteria by the same two reviewers independently. In case of double reporting, the article with the most complete data or largest sample was selected. Any disagreement between the two reviewers was resolved through discussion or by consulting a third reviewer (N.E.v.d.A.).
Data extraction and synthesis
Characteristics of the included studies were extracted by two reviewers independently (E.J., M.F.W.). A list of the extracted characteristics can be found in Appendix 2. Studies were categorized based on the main nutritional component or strategy. Any disagreement between the two reviewers was resolved through discussion or by consulting a third reviewer (N.E.v.d.A.).
Deviations from the protocol
Compared to the pre-registered protocol, the inclusion criteria were broadened in order to include both intervention and observation studies. The terms “milk” and “formula” were added to the search terms.
Results
Study selection
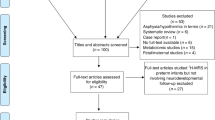
After duplicate removal, 11,057 records were screened, 66 full-text articles were assessed for eligibility, and 32 articles were included in this review (Fig. 1). No additional studies were obtained from screening the reference lists of the included studies. Seven studies assessed parenteral feeding duration, twelve studies assessed (composition of) human milk intake, fifteen studies assessed overall macronutrient and energy intake, four studies assessed fatty acid supplementation, and one study assessed glutamine supplementation in relation to white matter development in preterm infants. Some studies fitted multiple categories. An overview of the extracted study characteristics and key findings is provided in Tables 1 and 2.
White matter development measurements
Most studies used MRI to assess white matter volumes or surface, to score white matter maturation or injury (e.g., Kidokoro scoring), or to analyze white matter microstructure using diffusion MRI (dMRI). Increased fractional anisotropy (FA) and reduced mean and radial diffusivity (MD, RD) are indications of improved axonal development and myelination. Other dMRI measures include orientation dispersion and neurite density indexes (ODI, NDI), reflecting the organization and density of axons, respectively. Three studies used cranial ultrasound (cUS) to detect possible brain abnormalities early on, including PVL, and to measure the growth of the largest white matter structure in the brain: the corpus callosum.
Results: parenteral feeding duration
Seven observational studies evaluated the association between parenteral feeding duration and white matter development in preterm infants (Tables 1 and 2). Parenteral feeding duration was associated with an increase in white matter abnormality MRI score30 and reduced FA in several white matter tracts20,31 at TEA. Other studies showed no significant associations between parenteral feeding duration and diffuse white matter abnormality volume (DWMA)32,33,34 and dMRI metrics35 at TEA. None of the included studies assessed NDO in relation to parenteral feeding duration.
Results: human milk intake
Eleven observational studies and one case–control study evaluated the association between human milk intake and white matter development in preterm infants (Tables 1 and 2).
Five studies assessed white matter volumes in relation to own mother’s milk (MM) intake. Receiving mainly or exclusively MM during NICU stay was not significantly associated with white matter volume at TEA36,37 and 7 years of age.37 One study found a positive association between the percentage of expressed MM in diet and white matter volume at 16 years of age.38 Regarding MM composition, higher energy content was positively associated with white matter volumes at TEA.39 Two studies assessed DWMA volumes. Duration of MM intake was not significantly associated with DWMA volume at TEA.33 However, exclusive MM intake was associated with decreased DWMA volume at TEA, compared to exclusive formula milk (FM) intake,34 indicating a potential neuroprotective effect of MM.
Seven studies assessed dMRI metrics. At TEA, infants receiving mainly human milk (either MM or donor milk (DM)) showed lower MD values in white matter tracts compared to infants receiving mainly FM.40 Duration41 and amount19,20 of human milk intake was also positively associated with FA. One study even showed a dose-dependent effect of MM intake on FA, MD, and RD values.36 In another study, macronutrient and energy composition of MM was generally positively, but not significantly, associated with FA values.39 At 8.6 years, significant associations between MM intake and dMRI metrics were not present.42
Two studies investigated differences between MM, DM, and FM intake. Infants receiving mainly MM or DM did not differ significantly in white matter volume at TEA, compared to infants receiving mainly FM. Infants receiving mainly MM did not differ significantly in white matter volume and dMRI metrics at TEA, compared to infants receiving mainly DM or FM.40 At 8.6 years of age, infants receiving exclusively MM did also not differ significantly in dMRI metrics, compared to infants receiving MM supplemented with DM or FM.42 However, infants receiving mainly DM showed lower MD in several white matter tracts at TEA compared to infants receiving mainly FM.40
One study focused on intranasal administration of MM in very low birthweight infants with severe brain injury. Compared to a control group, supplementation of intranasal MM was associated with a lower incidence of cystic PVL at TEA.43
Finally, three studies assessed NDO. Human milk intake was not significantly associated with NDO at 2 years,20,37 but was positively associated with IQ performance at age 737 and 16 years, especially in boys.38
Results: macronutrient and energy intake
Twelve observational and three intervention studies evaluated associations between macronutrient and energy intake and white matter development in preterm infants (Tables 1 and 2). Two studies focused on the growth of the corpus callosum, which was positively associated with enteral protein and energy intake and negatively associated with parenteral protein and energy intake.44,45
Four studies assessed white matter volumes up to TEA. Total protein, lipid, carbohydrate, and energy intakes during the first 4 weeks of life were not significantly associated with white matter volumes.46,48,48 Another study, comparing two different nutrition protocols which differed in total protein and energy intake, found no differences in white matter volumes at TEA, except for the cingulate gyrus at 30 weeks postmenstrual age.49 On the contrary, a different study showed positive associations between protein intake at day seven of life (up to 4.5 g/kg/day) and WM surface area at TEA.50
Six studies assessed dMRI metrics up to TEA. Compared to a standard diet, infants receiving an enhanced diet, containing higher protein, lipid, and energy intakes, showed lower MD in several white matter tracts at TEA.18 Two other studies showed that total lipid and energy intakes, but not total and enteral protein intake, during the first 2–4 weeks of life were positively associated with FA in several white matter tracts.19,20 On the contrary, some other studies found total protein intake, but not total lipid and energy intake, to be positively associated with FA.50,51 When looking only at enteral intakes, multiple studies found positive associations for protein19,20,42,51 and carbohydrate intake19 in relation to FA.
The effect of macronutrient intake on childhood dMRI metrics was assessed in one study. Receiving enteral protein and lipid intakes, but not carbohydrate intakes, in amounts according to recommendations, was positively associated with dMRI metrics at 5.8 years, compared to receiving lower intakes.42 Other studies showed no significant associations between macronutrient and energy intake and Kidokoro scoring at TEA,52 T2 relaxation time at TEA,53 and thinning of the corpus callosum and immature myelination scores at 16 years of age.54
Six studies assessed NDO in relation to macronutrient and energy intake. Protein and energy deficits were associated with lower cognitive and motor scores at 3 months, but not at 9 months, corrected age.53 Total protein and energy intakes were positively associated with cognitive and motor scores at 2 years,20 although not in all studies.51 Another study indicates positive associations between total protein intake and IQ at 5 years.50 Compared to a standard diet, a high-nutrient diet was positively associated with IQ performance at 16 years of age.54 Two studies found no significant associations between nutritional intake and NDO.47,53
Results: fatty acid supplementation
Four intervention studies evaluated the association between docosahexaenoic and arachidonic acid (DHA/AA) supplementation and white matter development in preterm infants (Tables 1 and 2). Compared to infants receiving a formula without DHA/AA, infants receiving DHA/AA supplementation during the first 6 months of life did not differ significantly in myelination index at 3 and 12 months corrected age.55 Infants receiving the formula without DHA/AA even showed a trend toward higher motor and cognitive scores up to 2 years corrected age.55 High-dose DHA/AA supplementation (32 mg DHA and 31 mg AA per 100 mL during the first 9 weeks of life) did also not significantly influence white matter volume,56 dMRI metrics,57 or NDO56,57 at 8.6 years of age. However, another study showed that, compared to infants receiving a standard diet, infants receiving an enhanced diet containing DHA/AA during hospital stay had lower MD in several white matter tracts at TEA.18
Results: glutamine supplementation
The effect of glutamine supplementation on white matter development in preterm infants was tested in one single study (Tables 1 and 2). Compared to infants receiving a placebo, infants receiving glutamine supplementation during the first month of life showed increased white matter volumes and a trend toward higher FA in the bilateral cingulum hippocampal tract at 8.6 years of age.58 These findings were associated with a reduction in the number of serious neonatal infections.58 NDO was not assessed in this study.
Discussion
In this scoping review, 32 studies were included that assessed the influence of early postnatal nutrition on white matter development in preterm infants. Most studies used observational designs and MRI as main outcome measure. Neutral to negative associations were found between longer parenteral feeding duration and white matter development. Neutral to positive associations were found between macronutrient, energy, and human milk intake and white matter development, especially when fed enterally and in amounts according to recommendations. Results on fatty acid supplementation remain more inconclusive due to contradicting results. Results on glutamine supplementation seem promising, although these are based on only one study.
Parenteral feeding duration
Three out of seven studies suggest a negative influence of parenteral feeding duration on white matter development in preterm infants. Significant negative effects were most often observed in studies with large sample sizes assessing white matter microstructure20,30,31 compared to studies with smaller sample sizes35 and studies assessing DWMA volume.32,33,34 This negative association is likely confounded by the impaired clinical status of an infant, as the sickest infants receive parenteral nutrition for the longest duration.59,60 Nevertheless, parenteral feeding itself may also negatively influence white matter development by inducing side effects of aggressive parenteral nutrition, such as hyperglycemia, metabolic acidosis, and elevated urea and ammonia concentrations.61 Moreover, parenteral nutrition may result in undernourishment of choline, which may affect brain development.62 Parenteral feeding duration has previously been negatively associated with NDO,63 although improved survival due to parenteral nutrition may be a confounder.
In contrast, enteral intakes were positively associated with preterm brain development in most included studies.19,20,42,44,45,51 Similarly, this may be confounded by the fact that only the healthiest preterm infants are able to obtain recommended enteral intakes. Nevertheless, enteral intakes may also positively influence white matter development by reducing inflammation, stimulating insulin-like growth factor 1 (IGF-1) levels,64 and the microbiome–gut–brain axis.11
Human milk intake
Nine out of twelve studies suggest a positive influence of human milk intake on white matter development in preterm infants. Human milk contains many bioactive components and substrates, such as human milk oligosaccharides and fatty acids, that supports healthy gut microbiota65 and immune system development.66,67 Hence, human milk may reduce the negative impact of inflammation on white matter development. This is in line with two of the included studies, in which the positive association between MM intake and white matter development became weaker when controlling for infections.38,40
Three out of twelve studies found no significant associations. One of these studies had a small sample size and assessed white matter development at the age of 8.6 years.42 Although this study controlled for sex and birthweight, there are many other confounding factors up to 8.6 years, making it difficult to detect significant associations. Other studies measured MM intake duration during the first 4 weeks of life,33,37 which may be less informative than human milk amount,19,20,38 composition,39 and comparing human milk to FM intake.34,36,40 Although one study did find a significant association for human milk duration,41 this study looked at white matter microstructure, which may be a more sensitive measure than white matter (abnormality) volume measured in the two studies that did not find significant associations.
If no or insufficient MM is available, DM or FM are provided for enteral nutrition (exclusively or as a supplement). Compared to MM, DM contains lower levels of protein and functional components due to pasteurization and often being derived from mothers with term babies. As a supplement to MM or FM, DM has not proven superior to FM on brain development.40,42 Compared to FM, however, DM might benefit the immune system, as it is associated with lower rates of necrotizing enterocolitis,68 and may therefore better support white matter development. In an exploratory study, preterm infants receiving mainly DM showed some improved microstructure at TEA compared to infants receiving mainly FM.40 However, additional studies are warranted.
In line with a previous review,69 we failed to find any positive association between MM intake and NDO at 2 years,20,37 but we did find positive associations between MM intake and IQ performance into childhood37 and adolescence.38 Although this might reflect the influence of breastfeeding-related confounders over time, including more skin-to-skin contact70 and higher socio-economic status,71 most studies controlled for socio-economic status. In summary, our results suggest a positive influence of MM on white matter and long-term development in preterm infants.
Macronutrient and energy intake
Ten out of fifteen studies suggest a positive influence of increased total and enteral macronutrient and energy intake on white matter development in preterm infants. In general, lipids and carbohydrates are essential to provide sufficient energy and fatty acids for brain growth. Protein is important for providing building blocks and having roles as enzymes, transport proteins, and hormones, such as IGF-1,72 which is important for brain development.48,73 However, several studies have demonstrated that excessive amounts of protein may cause harm, particularly if energy and micronutrient supplies are not met.
Five out of fifteen studies found no significant association between macronutrient and energy intake and white matter development. This may be due to several factors. Firstly, observational study designs46,48,48,52 and outcome measures beyond childhood54 increase the influence of confounding factors. Secondly, low inter-individual variations in nutrient intake compromise the detection of significant associations. For example, over the course of 28 days, there was only a difference of 100 calories between the intervention and control group in the study by Tan et al. (2008). Thirdly, nutritional intake should be high enough to support brain development. However, recommended intakes after birth are often not achieved in preterm infants.74 For example, in one study, only 3.4% of preterm infants met recommended protein intakes.47 This cannot be solved by simply increasing or prolonging parenteral intakes, as this seems negatively associated with white matter development.44,45 Our results suggest that enteral intake of protein, lipid, and energy in amounts according to recommendations is required to support white matter development into childhood.42
Previous reviews suggest that optimized macronutrient intake can improve NDO in preterm infants.22,75 Our results indicate neutral to negative associations between protein intakes below recommendations and NDO.47,51,53 One study did find a positive association between total protein and energy intakes below recommendations and NDO,20 but this was of relatively small effect size. Isaacs et al. (2008) found a positive association between high-nutrient intake and IQ scores at 16 years, although the reported confidence interval was contradictory to the p value. In a large population-based study, total protein intake above recommendations (up to 4.50 g/kg) on day seven of life was positively associated with survival and full-scale IQ scores at 5 years of age.50 However, high parenteral protein intake (4.0 g/kg/day) on the first day of life was associated with lower head circumference percentiles in another study.76 Hence, macronutrient intake according to recommendations is challenging, but might support NDO in preterm infants if provided gradually.
Fatty acid supplementation
Human milk contains a high amount of long-chain poly-unsaturated fatty acids (LC-PUFAs), such as DHA and AA. DHA and AA modulate immune responses77 and are important constituents of phospholipids, thereby supporting neuronal membrane synthesis.78 In the early 1990s, post-mortem studies already showed positive associations between human milk intake and DHA concentrations in the brain.79,80 Moreover, preterm infants are highly dependent on dietary LC-PUFA intake, as they are born prior to fetal accretion of LC-PUFAs.81 This suggests a potential benefit of LC-PUFAs supplementation in preterm infants.
Four intervention studies evaluated the influence of DHA/AA supplementation on white matter development in preterm infants. An enhanced diet containing, among others, higher DHA and AA seemed to improve white matter integrity at TEA.18 Nevertheless, DHA/AA supplementation alone did not improve white matter development or NDO at 3 months, 12 months (15 mg DHA and 31 mg AA per 100 mL FM), and 8.6 years of age (32 mg DHA and 31 mg AA per 100 mL of human milk).55,57,57 This is in contrast to two previous studies, showing a positive association between DHA serum levels and white matter development.82,83
These inconclusive findings may be due to several factors. Firstly, the studies differed in dosing, sample size, and outcome measure. Secondly, some studies were prone to confounding as outcomes were measured at 8.6 years of age.56,57 Thirdly, some studies excluded preterm infants with brain damage55,57,57 and infants that did not receive any human milk,56,57 while especially these vulnerable infants might benefit from fatty acid supplementation. Moreover, high dosing, up to 60 mg/kg/day, may be needed to compensate for DHA oxidation, deficits, and intestinal malabsorption in the preterm infant.81 Longer supplementation duration has previously been shown to improve NDO,84,85 but other factors such as sex,86 fatty acid oil source (e.g., fish oil, krill oil, egg),87 and DHA/AA ratio88,89 also play a role. In addition, studies on DHA serum levels83,90 found that near-birth DHA levels were more strongly associated with brain development compared to near-term DHA levels, suggesting that DHA levels should be increased much earlier, possibly antenatally. In summary, more research is needed on fatty acid supplementation in preterm infants to best understand the optimal timing and intervention to optimize long-term outcomes.
Glutamine supplementation
The results suggest a positive influence of glutamine supplementation on white matter development in preterm infants. Glutamine is a conditionally essential amino acid in preterm infants important for functional gut integrity, thereby decreasing the chance of serious neonatal infections and subsequent white matter injury.91 Furthermore, glutamine is an important substrate for rapidly proliferating cells and influences both cell proliferation and growth.92 Therefore, the effect of glutamine may be more growth-mediated compared to other nutrients, such as fatty acids, that act upon microstructural membrane formation. Indeed, glutamine supplementation was more strongly associated with an increase in head circumference and white matter volume58,93 compared to changes in dMRI metrics.58
However, the studies discussed above are based on only one cohort and follow-up studies indicate no significant influence of glutamine supplementation on NDO at 294 and 7.5 years95 of age. In addition, a meta-analysis indicates no effect of glutamine supplementation on mortality, morbidity, and NDO in preterm infants,96 making it difficult to draw final conclusions.
Research gaps and recommendations for future research
Future studies should provide detailed information on nutritional intake to improve study quality and determine any supplementation needed. This can be done by taking inter-individual variation in standard nutritional intake (e.g., enteral vs. parenteral intake, MM, DM, and/or FM intake), MM composition, and fortification products into account.97,98 In addition, standardized reporting of nutritional intake99 should be used to improve comparison among studies.
Interestingly, significant associations between nutritional intake and white matter development were more often detected at the microstructural level using dMRI compared to volumetric MRI measures. As Beauport et al. (2017) suggested, it might be that white matter develops mostly through increased microstructural complexity, while gray matter develops mostly through growth and expansion. Indeed, associations between early nutrient intake and volumetric gray matter development were more often detected compared to volumetric white matter development.44,46,49,52 Therefore, future studies should use quantitative neuroimaging techniques to assess white matter development.
Future studies should also use interventional designs to increase power. If controlled single-nutrient supplementation studies are performed, they should use different dosing, duration, and timing to determine beneficial supplementation strategies. However, care should be taken when supplementing a single nutrient, because unbalanced nutrient supply may cause harm. Also, unbalanced, high amino acid supply can make other amino acids deficient and increase amino acid plasma concentrations.74 Combined nutrient supplementation to target specific neurodevelopmental processes may be more effective.100 Supplementation of multiple phospholipid precursors has previously been shown to improve neuronal membrane formation,101 white matter102 and cognitive development103,104 in preterm infants. Also, supplementation of pre- and probiotics to support healthy gut colonization and white matter development in preterm infants is receiving increased attention.105,106 Although intervention studies with human milk are more difficult, as it is unethical to withhold infants from breastfeeding, it is possible to randomize intranasal administration of MM43 and breastfeeding promotion interventions between hospitals.107
If possible, future studies should use large sample sizes to increase power and to control for multiple confounders simultaneously, as multiple perinatal risk factors additively increase the chance of white matter injury.31 Ideally, a fetal or term-born control group is used as a reference to better interpret effects on brain development.108 In addition, studies should be powered to assess white matter development for both sexes separately, as MM composition, the influence of nutritional interventions,109 and volumetric white matter development110 differs between girls and boys. NDO assessment should be standardized, hypothesis driven, and targeted at the neurodevelopmental domain that is expected to be influenced.22,75,111 This will improve the power to detect meaningful associations between nutritional intake and NDO.
Strengths and limitations of this review
This scoping review is the first to summarize all published research on the influence of early postnatal nutrition on white matter development in preterm infants. In comparison to systematic reviews, scoping reviews aim to address broader topics and are less dependent on detailed research questions or quality assessments.112 Therefore, this review was able to answer a broad question to guide future research. Nevertheless, heterogeneity among study designs makes it difficult to compare studies and the reviewing process may be subject to publication and reporting biases.113
Conclusion
Optimizing early postnatal nutrition can positively influence white matter development and subsequent NDO in preterm infants. More studies are needed with large sample sizes using quantitative neuroimaging techniques and interventional designs controlling for confounders to define optimal nutritional intakes in preterm infants.
References
Ortinau, C. & Neil, J. The neuroanatomy of prematurity: normal brain development and the impact of preterm birth. Clin. Anat. 28, 168–183 (2015).
Pascal, A. et al. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev. Med. Child Neurol. 60, 342–355 (2018).
Wilson, S. et al. Development of human white matter pathways in utero over the second and third trimester. Proc. Natl Acad. Sci. USA 118, 1–7 (2021).
van Tilborg, E. et al. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia 66, 221–238 (2018).
Back, S. A. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol 134, 331–349 (2017).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Cayam-Rand, D. et al. Predicting developmental outcomes in preterm infants: a simple white matter injury imaging rule. Neurology 93, E1231–E1240 (2019).
Back, S. A. & Miller, S. P. Brain injury in premature neonates: a primary cerebral dysmaturation disorder? Ann. Neurol. 75, 469–486 (2014).
Elitt, C. M. & Rosenberg, P. A. The challenge of understanding cerebral white matter injury in the premature infant. Neuroscience 276, 216–238 (2014).
Cusick, S. E. & Georgieff, M. K. The role of nutrition in brain development: the golden opportunity of the “First 1000 Days”. J. Pediatr. 175, 16–21 (2016).
Keunen, K., Van Elburg, R. M., Van Bel, F. & Benders, M. J. N. L. Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr. Res. 77, 148–155 (2015).
Volpe, J. J. Dysmaturation of premature brain: importance, cellular mechanisms, and potential interventions. Pediatr. Neurol. 95, 42–66 (2019).
Fink, N. H., Collins, C. T., Gibson, R. A., Makrides, M. & Penttila, I. A. Targeting inflammation in the preterm infant: the role of the omega-3 fatty acid docosahexaenoic acid. J. Nutr. Intermed. Metab. 5, 55–60 (2016).
Garofalo, R. Cytokines in human milk. J. Pediatr. 156, S36–S40 (2010).
Eyles, D., Burne, T. & Mcgrath, J. Vitamin D in fetal brain development. Semin. Cell Dev. Biol. 22, 629–636 (2011).
Jarosz, M., Olbert, M., Wyszogrodzka, G., Młyniec, K. & Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 25, 11–24 (2017).
Georgieff, M. K. Nutrition and the developing brain: nutrient priorities and measurement. Am. J. Clin. Nutr. 85, 614–620 (2007).
Strømmen, K. et al. Enhanced nutrient supply to very low birth weight infants is associated with improved white matter maturation and head growth. Neonatology 107, 68–75 (2015).
Schneider, J. et al. Nutrient intake in the first two weeks of life and brain growth in preterm neonates. Pediatrics 141, e20172169 (2018).
Coviello, C. et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr. Res. 83, 102–110 (2018).
Mudd, A. T. & Dilger, R. N. Early-life nutrition and neurodevelopment: use of the piglet as a translational model. Adv. Nutr. 8, 92–104 (2017).
Chan, S. H. T., Johnson, M. J., Leaf, A. A. & Vollmer, B. Nutrition and neurodevelopmental outcomes in preterm infants: a systematic review. Acta Paediatr. 105, 587–599 (2016).
Belfort, M. B. & Ehrenkranz, R. A. Neurodevelopmental outcomes and nutritional strategies in very low birth weight infants. Semin. Fetal Neonatal Med. 22, 42–48 (2017).
Ottolini, K. M., Andescavage, N., Keller, S. & Limperopoulos, C. Nutrition and the developing brain: the road to optimizing early neurodevelopment: a systematic review. Pediat. Res. 87, 194–201 (2020)
Hortensius, L. M., Van Elburg, R. M., Nijboer, C. H., Benders, M. J. N. L. & De Theije, C. G. M. Postnatal nutrition to improve brain development in the preterm infant: A systematic review from bench to bedside. Front. Physiol. 10, 1–18 (2019).
Peters, M.D.J. et al. Chapter 11: Scoping Reviews (2020 version). In: Aromataris E, Munn Z Editors. JBI Manual for Evidence Synthesis, JBI, 2020
Tricco, A. C. et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 169, 467–473 (2018).
Mueen Ahmed, K. K. & Al Dhubaib, B. E. Zotero: a bibliographic assistant to researcher. J. Pharm. Pharmacother. 2, 303–305 (2011).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 5, 1–10 (2016).
Brouwer, M. J. et al. Preterm brain injury on term-equivalent age MRI in relation to perinatal factors and neurodevelopmental outcome at two years. PLoS One 12, 1–13 (2017).
Barnett, M. L. et al. Exploring the multiple-hit hypothesis of preterm white matter damage using diffusion MRI. Neuroimage Clin. 17, 596–606 (2018).
Parikh, N. A., Lasky, R. E., Kennedy, K. A., McDavid, G. & Tyson, J. E. Perinatal factors and regional brain volume abnormalities at term in a cohort of extremely low birth weight infants. PLoS One 8, e62804 (2013).
Parikh, N. A., He, L., Li, H., Priyanka Illapani, V. S. & Klebanoff, M. A. Antecedents of objectively diagnosed diffuse white matter abnormality in very preterm infants. Pediatr. Neurol. 106, 56–62 (2020).
Parikh, N. A. et al. Perinatal risk and protective factors in the development of diffuse white matter abnormality on term-equivalent age magnetic resonance imaging in infants born very preterm. J. Pediatr. 233, 58–65.e3 (2021).
Rogers, CE., Smyser, T., Smyser, CD., Shimony, J., Inder, TE. & Neil, JJ. Regional white matter development in very preterm infants: perinatal predictors and early developmental outcomes. Physiol. Behav. 176, 100–106 (2016).
Blesa, M. et al. Early breast milk exposure modifies brain connectivity in preterm infants. Neuroimage 184, 431–439 (2019).
Belfort, M. B. et al. Breast milk feeding, brain development, and neurocognitive outcomes: a 7-year longitudinal study in infants born at less than 30 weeks’ gestation. J. Pediatr. 177, 133–139.e1 (2016).
Isaacs, E. B. et al. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr. Res. 67, 357–362 (2010).
Bell, K. A. et al. Associations of macronutrient intake determined by point-of-care human milk analysis with brain development among very preterm infants. Children (Basel) 9, 969 (2022).
Ottolini, K. M., Andescavage, N., Kapse, K., Jacobs, M. & Limperopoulos, C. Improved brain growth and microstructural development in breast milk–fed very low birth weight premature infants. Acta Paediatr. 109, 1580–1587 (2020).
Pogribna, U. et al. Perinatal clinical antecedents of white matter microstructural abnormalities on diffusion tensor imaging in extremely preterm infants. PLoS One 8, e72974 (2013).
Sato, J. et al. Early nutrition and white matter microstructure in children born very low birth weight. Brain Commun. 3, 1–12 (2021).
Keller, T. et al. Intranasal breast milk for premature infants with severe intraventricular hemorrhage—an observation. Eur. J. Pediatr. 178, 199–206. https://doi.org/10.1007/s00431-018-3279-7 (2019).
Terrin, G. et al. Early protein intake influences neonatal brain measurements in preterms: an observational study. Front. Neurol. 11, 885 (2020).
Boscarino, G. et al. Effects of early energy intake on neonatal cerebral growth of preterm newborn: an observational study. Sci. Rep. 11, 1–7 (2021).
Ottolini, K. M. et al. Early lipid intake improves cerebellar growth in very low-birth-weight preterm infants. J. Parenter. Enter. Nutr. 45, 587–595 (2021).
Power, V. A. et al. Nutrition, growth, brain volume, and neurodevelopment in very preterm children. J. Pediatr. 215, 50–55.e3 (2019).
Hansen-Pupp, I. et al. Postnatal decrease in circulating insulin-like growth factor-I and low brain volumes in very preterm infants. J. Clin. Endocrinol. Metab. 96, 1129–1135 (2011).
van Beek, P. E. et al. Increase in brain volumes after implementation of a nutrition regimen in infants born extremely preterm. J. Pediatr. 223, 57–63.e5 (2020).
Rozé, J. C. et al. Association between early amino acid intake and full-scale IQ at age 5 years among infants born at less than 30 weeks’ gestation. JAMA Netw. Open 4, e2135452. https://doi.org/10.1001/jamanetworkopen.2021.35452 (2021).
Hortensius, L. M. et al. Nutritional intake, white matter integrity, and neurodevelopment in extremely preterm born infants. Nutrients 13, 1–14 (2021).
Beauport, L. et al. Impact of early nutritional intake on preterm brain: a magnetic resonance imaging study. J. Pediatr. 181, 29–36.e1 (2017).
Tan, M., Abernethy, L. & Cooke, R. Improving head growth in preterm infants – a randomised controlled trial II: MRI and developmental outcomes in the first year. Arch. Dis. Child Fetal Neonatal Ed. 93, 342–346 (2008).
Isaacs, E. B. et al. The effect of early human diet on caudate volumes and IQ. Pediatr. Res. 63, 308–314 (2008).
Van Wezel-Meijler, G. et al. Dietary supplementation of long-chain polyunsaturated fatty acids in preterm infants: effects on cerebral maturation. Acta Paediatr. 91, 942–950 (2002).
Almaas, A. N. et al. Long-chain polyunsaturated fatty acids and cognition in VLBW infants at 8 years: An RCT. Pediatrics 135, 972–980 (2015).
Almaas, A. N. et al. Diffusion tensor imaging and behavior in premature infants at 8 years of age, a randomized controlled trial with long-chain polyunsaturated fatty acids. Early Hum. Dev. 95, 41–46 (2016).
De Kieviet, J. F. et al. Effects of glutamine on brain development in very preterm children at school age. Pediatrics 130, e1121–e1127 (2012).
Ehrenkranz, R. A. et al. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr. Res. 69, 522–529 (2011).
Moltu, S. J. et al. Nutritional management of the critically ill neonate: a position paper of the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 73, 274–289 (2021).
Stensvold, H. J. et al. Early enhanced parenteral nutrition, hyperglycemia, and death among extremely low-birth-weight infants. JAMA Pediatr. 169, 1003–1010 (2015).
Nilsson, A. K. et al. Serum choline in extremely preterm infants declines with increasing parenteral nutrition. Eur. J. Nutr. 60, 1081–1089 (2021).
Asztalos, E. V., Church, P. T., Riley, P., Fajardo, C. & Shah, P. S. Neonatal factors associated with a good neurodevelopmental outcome in very preterm infants. Am. J. Perinatol. 34, 388–396 (2017).
Wojnar, M. M., Fan, J., Li, Y. H. & Lang, C. H. Endotoxin-induced changes in IGF-I differ in rats provided enteral vs. parenteral nutrition. Am. J. Physiol. 276, E455–E464 (1999).
Marcobal, A. et al. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 58, 5334–5340 (2010).
Ayechu-Muruzabal, V. et al. Diversity of human milk oligosaccharides and effects on early life immune development. Front. Pediatr. 6, 1–9 (2018).
Kotey, F. O. & Spatz, D. L. White matter injury in preterm infants: could human milk play a role in its prevention? Adv. Neonatal Care 13, 89–94 (2013).
Quigley, M., Embleton, N. D. & McGuire, W. Formula versus maternal breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 8, CD002972 (2019).
Chetta, K. E., Schulz, E. V. & Wagner, C. L. Outcomes improved with human milk intake in preterm and full-term infants. Semin. Perinatol. 45, 151384 (2021).
Dueñas-Espín, I. et al. Breastfeeding education, early skin-to-skin contact and other strong determinants of exclusive breastfeeding in an urban population: a prospective study. BMJ Open 11, 1–8 (2021).
Walfisch, A., Sermer, C., Cressman, A. & Koren, G. Breast milk and cognitive development-the role of confounders: a systematic review. BMJ Open 3, e003259 (2013).
Yumani, D. F. J., Calor, A. K. & van Weissenbruch, M. M. The course of IGF-1 levels and nutrient intake in extremely and very preterm infants during hospitalisation. Nutrients 12, 1–13 (2020).
Okuma, C. et al. Microstructural brain and multivoxel spectroscopy in very low birth weight infants related to insulin-like growth factor concentration and early growth. Horm. Res. Paediatr. 79, 197–207 (2013).
Hay, W. W. Aggressive nutrition of the preterm infant. Curr. Pediatr. Rep. 1. https://doi.org/10.1007/s40124-013-0026-4 (2013).
Cormack, B. E., Harding, J. E., Miller, S. P. & Bloomfield, F. H. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: a narrative review. Nutrients 11, 2029 (2019).
Balakrishnan, M. et al. Growth and neurodevelopmental outcomes of early high‐dose parenteral amino acid intake in very low birth weight infants: a randomized controlled trial. JPEN J Parenter Enteral Nutr. 42, 597–606 (2017)
Lapillonne, A. & Moltu, S. J. Long-chain polyunsaturated fatty acids and clinical outcomes of preterm infants. Ann. Nutr. Metab. 69, 36–44 (2016).
Sakamoto, T., Cansev, M. & Wurtman, R. J. Oral supplementation with docosahexaenoic acid and uridine-5′-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. 1182, 50–59 (2007).
Farquharson, J., Cockburn, F., Patrick, W. A., Jamieson, E. C. & Logan, R. W. Infant cerebral cortex phospholipid fatty-acid composition and diet. Lancet 340, 810–813 (1992).
Makrides, M., Neumann, M. A., Byard, R. W., Simmer, K. & Gibson, R. A. Fatty acid composition of brain, retina, and erythrocytes in breast-and formula-fed infants. Am. J. Clin. Nutr. 60, 189–194 (1994).
Lapillonne, A., Groh-Wargo, S., Lozano Gonzalez, C. H. & Uauy, R. Lipid needs of preterm infants: updated recommendations. J. Pediatr. 162, S37–S47 (2013).
Hortensius, L. M. et al. Serum docosahexaenoic acid levels are associated with brain volumes in extremely preterm born infants. Pediatr. Res. 90, 1177–1185. https://doi.org/10.1038/s41390-021-01645-w (2021).
Tam, E. W. Y. et al. Early postnatal docosahexaenoic acid levels and improved preterm brain development. Pediatr. Res. 79, 723–730 (2016).
Fang, P. C. et al. The effect of supplementation of docosahexaenoic acid and arachidonic acid on visual acuity and neurodevelopment in larger preterm infants. Chang Gung Med. J. 28, 708–715 (2005).
Clandinin, M. T. et al. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J. Pediatr. 146, 461–468 (2005).
Makrides, M. et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid – a randomized controlled. Trial 301, 175–182 (2015).
Sugasini, D., Yalagala, P. C. R., Goggin, A., Leon M. Tai, P. & Subbaiah, P. V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 74, 108231. https://doi.org/10.1159/000444169.Carotid (2019).
Klevebro, S., Juul, S. E. & Wood, T. R. A more comprehensive approach to the neuroprotective potential of long-chain polyunsaturated fatty acids in preterm infants is needed—should we consider maternal diet and the n-6:n-3 fatty acid ratio? Front. Pediatr. 7, 533 (2020).
Alshweki, A. et al. Effects of different arachidonic acid supplementation on psychomotor development in very preterm infants; a randomized controlled trial. Nutr. J. 14, 101 (2015).
Kamino, D. et al. Postnatal polyunsaturated fatty acids associated with larger preterm brain tissue volumes and better outcomes. Pediatr. Res. 83, 93–101 (2018).
Van Den Berg, A., Van Elburg, R. M., Westerbeek, E. A. M., Twisk, J. W. R. & Fetter, W. P. F. Glutamine-enriched enteral nutrition in very-low-birth-weight infants and effects on feeding tolerance and infectious morbidity: a randomized controlled trial. Am. J. Clin. Nutr. 81, 1397–1404 (2005).
Mok, E. & Hankard, R. Glutamine supplementation in sick children: is it beneficial? J. Nutr. Metab. 2011, 617597 (2011).
De Kieviet, J. F. et al. Glutamine effects on brain growth in very preterm children in the first year of life. Clin. Nutr. 33, 69–74 (2014).
van Zwol, A. et al. Neurodevelopmental outcomes of very low‐birth‐weight infants after enteral glutamine. Acta Paediatr. 97, 562–7 (2008)
De Kieviet, J. F. et al. Effects of neonatal enteral glutamine supplementation on cognitive, motor and behavioural outcomes in very preterm and/or very low birth weight children at school age. Br. J. Nutr. 108, 2215–2220 (2012).
Moe-Byrne, T., Brown, J. V. E. & Mcguire, W. Glutamine supplementation to prevent morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2016, CD001457 (2016).
Rochow, N. et al. Individualized target fortification of breast milk with protein, carbohydrates, and fat for preterm infants: a double-blind randomized controlled trial. Clin. Nutr. 40, 54–63 (2021).
Fusch, S. et al. Individualized target fortification of breast milk: optimizing macronutrient content using different fortifiers and approaches. Front. Nutr. 8, 652641 (2021).
Cormack, B. E., Embleton, N. D., Van Goudoever, J. B., Hay, W. W. & Bloomfield, F. H. Comparing apples with apples: it is time for standardized reporting of neonatal nutrition and growth studies. Pediatr. Res. 79, 810–820 (2016).
Hauser, J., Sultan, S., Rytz, A., Steiner, P. & Schneider, N. A blend containing docosahexaenoic acid, arachidonic acid, vitamin B12, vitamin B9, iron and sphingomyelin promotes myelination in an in vitro model. Nutr. Neurosci. 23, 931–945 (2020).
Holguin, S., Martinez, J., Chow, C. & Wurtman, R. Dietary uridine enhances the improvement in learning and memory produced by administering DHA to gerbils. FASEB J. 22, 3938–3946 (2008).
Schneider, N. et al. A nutrient formulation affects developmental myelination in term infants: a randomized clinical trial. Front. Nutr. 9, 1–12 (2022).
Andrew, M. J. et al. Neurodevelopmental outcome of nutritional intervention in newborn infants at risk of neurodevelopmental impairment: the Dolphin neonatal double-blind randomized controlled trial. Dev. Med. Child Neurol. 60, 897–905 (2018).
Andrew, M. J. et al. Nutritional intervention and neurodevelopmental outcome in infants with suspected cerebral palsy: the Dolphin infant double-blind randomized controlled trial. Dev. Med. Child Neurol. 60, 906–913 (2018).
Hortensius, L. M. et al. NutriBrain: protocol for a randomised, double-blind, controlled trial to evaluate the effects of a nutritional product on brain integrity in preterm infants. BMC Pediatr. 21, 1–10 (2021).
He, Y., Zhang, Y., Li, F. & Shi, Y. White matter injury in preterm infants: pathogenesis and potential therapy from the aspect of the gut–brain axis. Front. Neurosci. 16, 849372 (2022).
Kramer, M. S. et al. Breastfeeding and child cognitive development: new evidence from a large randomized. Trial 65, 578–584 (2008).
Sullivan, G. et al. Breast milk exposure is associated with cortical maturation in preterm infants Gemma. Ann. Neurol. 93, 591–603. https://doi.org/10.1002/ana.26559 (2023).
Tottman, A. C., Oliver, C. J., Alsweiler, J. M. & Cormack, B. E. Do preterm girls need different nutrition to preterm boys? Sex-specific nutrition for the preterm infant. Pediatr. Res. 89, 313–317 (2021).
Reiss, A. L. et al. Sex differences in cerebral volumes of 8-year-olds born preterm. J. Pediatr. 145, 242–249 (2004).
Schneider, N. & Garcia-Rodenas, C. L. Early nutritional interventions for brain and cognitive development in preterm infants: a review of the literature. Nutrients 9, 187 (2017).
Munn, Z. et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 18, 1–7 (2018).
Hopewell, S., Loudon, K., Clarke, M. J., Oxman, A. D. & Dickersin, K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst. Rev. 2009, MR000006. https://doi.org/10.1002/14651858.MR000006.pub3 (2009).
Acknowledgements
We thank the following staff at the University Library Utrecht for their help in drafting the initial search strategy and assistance in updating the initial search: Paulien Wiersma and Dr Marie-Louise S. Goudeau.
Funding
E.J. and M.J.N.L.B. are funded by the Athena Grant “Utrecht Center for Food and Health – research program specialized nutrition,” subsidy from the Dutch Ministry of Economic Affairs, Utrecht Province and the municipality of Utrecht. E.J., N.E. and M.J.N.L.B. are also funded by a Health-Holland-TKI grant, LSHM19087.
Author information
Authors and Affiliations
Consortia
Contributions
E.J., M.F.W., P.E.v.B., J.D., R.M.v.E., and N.E.v.d.A. made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data. E.J., P.E.v.B., J.D., R.M.v.E., L.M.H., E.W.Y.T., M.S.d.P., A.L., C.G.M.d.T., and N.E.v.d.A. contributed to drafting the article or revising it critically for important intellectual content. E.J., M.F.W., P.E.v.B., J.D., R.M.v.E., L.M.H., E.W.Y.T., M.S.d.P., A.L., C.G.M.d.T., M.J.N.L.B., N.E.v.d.A., S.J.M., G.Z., M.J.J. C.F., and S.I. contributed to final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Janson, E., Willemsen, M.F., Van Beek, P.E. et al. The influence of nutrition on white matter development in preterm infants: a scoping review. Pediatr Res (2023). https://doi.org/10.1038/s41390-023-02622-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-023-02622-1