Abstract
Background
Bone modifying agents (BMAs) prevent skeletal related events among patients with metastatic, castration-resistant prostate cancer (mCRPC) involving bone and prevent osteoporotic fractures among patients at high risk. BMA utilization for patients with mCRPC has not been well quantified.
Methods
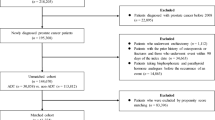
We used linked SEER registry and Medicare claims data. We included men diagnosed with stage IV prostate adenocarcinoma during 2007–2015, aged > = 66 at diagnosis, with sufficient continuous enrollment in Medicare Parts A, B, and D, who received androgen deprivation therapy. We limited to those who subsequently received a CRPC-defining treatment (CDT). We identified patients with evidence of bone metastasis using claims. Our primary outcome was receipt of a BMA (zoledronic acid or denosumab) within 180 days of initiating CDT.
Results
Among 1292 included patients, 1034 (80%) had bone metastasis. BMA use within 180 days of initiating CDT was higher among patients with bone metastases than those without (705/1034 [68%] vs 56/258 [22%]). Among patients without bone metastasis, those with high osteoporotic fracture risk were more likely than those without to receive a BMA (OR = 2.48, 95% CI: 1.17, 5.29); however, only 26% of patients with high fracture risk received a BMA. Among patients who received BMAs, most (62%) first initiated them >90 days before initiating CDT.
Conclusions
Two-thirds of patients with mCRPC and bone metastases received BMAs within 180 days after initiating CDT. A greater proportion of patients without bone metastasis may warrant BMA therapy for osteoporotic fracture prevention. Some patients with bone metastasis may be able to delay BMA initiation until CRPC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data underlying this article were provided by the Centers for Medicare and Medicaid Services (CMS) under license. Data are available from CMS on request and establishment of data use agreement.
Code availability
Code available in supplementary materials.
References
Oster G, Lamerato L, Glass AG, Richert-Boe KE, Lopez A, Chung K. et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer J Multinatl Assoc Support Care Cancer. 2013;21:3279–86. https://doi.org/10.1007/s00520-013-1887-3.
Baek YH, Jeon HL, Oh IS, Yang H, Park J, Shin JY. Incidence of skeletal-related events in patients with breast or prostate cancer-induced bone metastasis or multiple myeloma: A 12-year longitudinal nationwide healthcare database study. Cancer Epidemiol. 2019;61:104–10. https://doi.org/10.1016/j.canep.2019.05.013.
Broder MS, Gutierrez B, Cherepanov D, Linhares Y. Burden of skeletal-related events in prostate cancer: unmet need in pain improvement. Support Care Cancer J Multinatl Assoc Support Care Cancer. 2015;23:237–47. https://doi.org/10.1007/s00520-014-2437-3.
NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer, Version 4.2019. Published online August 19, 2019. Accessed January 17, 2020. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R. et al. Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference APCCC 2017. Eur Urol. 2018;73:178–211. https://doi.org/10.1016/j.eururo.2017.06.002.
Camacho PM. American association of clinical Endocrinologists/American college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pr J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2020;26:1–46. https://doi.org/10.4158/GL-2020-0524SUPPL.
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S. et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Min Res J Am Soc Bone Min Res. 2014;29:2520–6. https://doi.org/10.1002/jbmr.2269.
Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N. Engl J Med. 2005;352:154–64. https://doi.org/10.1056/NEJMoa041943.
Giri S, Zhu W, Wang R, Zeidan A, Podoltsev N, Gore SD. et al. Underutilization of guideline‐recommended supportive care among older adults with multiple myeloma in the United States. Cancer. 2019;125:4084–95. https://doi.org/10.1002/cncr.32428.
Zhou J, Sweiss K, Patel PR, Nutescu E, Ko N, Chiu BCH. et al. Racial differences in intravenous bisphosphonate utilization among older patients with multiple myeloma. J Clin Oncol. 2019;37:e18165–e18165. https://doi.org/10.1200/JCO.2019.37.15_suppl.e18165.
Lepisto EM. Concordance to National Comprehensive Cancer Network (NCCN) clinical practice guideline (GL) drug therapy recommendations for metastatic breast cancer: An analysis from the commercial managed care claims database PharMetrics (PM). J Clin Oncol. 2010;28:1032–1032. https://doi.org/10.1200/jco.2010.28.15_suppl.1032.
Liede A, Wade S, Lethen J, Hernandez RK, Warner D, Abernethy AP. et al. An observational study of concomitant use of emerging therapies and denosumab or zoledronic acid in prostate cancer. Clin Ther. 2018;40:536–49.e3. https://doi.org/10.1016/j.clinthera.2017.12.015.
Hagiwara M, Delea TE, Cong Z, Chung K. Utilization of intravenous bisphosphonates in patients with bone metastases secondary to breast, lung, or prostate cancer. Support Care Cancer J Multinatl Assoc Support Care Cancer. 2014;22:103–13. https://doi.org/10.1007/s00520-013-1951-z.
Oster G, Lamerato L, Glass AG, Richert-Boe KE, Lopez A, Chung K. et al. Use of intravenous bisphosphonates in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer J Multinatl Assoc Support Care Cancer. 2014;22:1363–73. https://doi.org/10.1007/s00520-013-2094-y.
Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet Lond Engl. 2011;377:813–22. https://doi.org/10.1016/S0140-6736(10)62344-6.
Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA. et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer Oxf Engl 1990. 2012;48:3082–92. https://doi.org/10.1016/j.ejca.2012.08.002.
Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J. et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol J Am Soc Clin Oncol. 2011;29:1125–32. https://doi.org/10.1200/JCO.2010.31.3304.
Autio KA, Farooki A, Glezerman IG, Chan A, Schneider CW, Barr HC. et al. Severe hypocalcemia associated with denosumab in metastatic castration-resistant prostate cancer: risk factors and precautions for treating physicians. Clin Genitourin Cancer. 2015;13:e305–9. https://doi.org/10.1016/j.clgc.2014.11.008.
Reed SD, Radeva JI, Glendenning GA, Saad F, Schulman KA. Cost-effectiveness of zoledronic acid for the prevention of skeletal complications in patients with prostate cancer. J Urol. 2004;171:1537–42. https://doi.org/10.1097/01.ju.0000116777.94426.60.
Snedecor SJ, Carter JA, Kaura S, Botteman MF. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a cost-effectiveness analysis. J Med Econ. 2013;16:19–29. https://doi.org/10.3111/13696998.2012.719054.
Kanellias N, Gavriatopoulou M, Terpos E, Dimopoulos MA. Management of multiple myeloma bone disease: impact of treatment on renal function. Expert Rev Hematol. 2018;11:881–8. https://doi.org/10.1080/17474086.2018.1531702.
Mitchell AP, Mishra A, Panageas KS, Lipitz-Snyderman A, Bach PB, Morris MJ. Real-world use of bone modifying agents in metastatic castration-sensitive prostate cancer. J Natl Cancer Inst. 2021:djab196. https://doi.org/10.1093/jnci/djab196
Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67.
Jain R, Bach P. Hospital Outpatient versus Physician Office Cost for Physician Administered Cancer Drugs. Memorial Sloan-Kettering Cancer Center; 2017. Accessed October 14, 2021. https://www.drugpricinglab.org/wp-content/uploads/2017/01/Hospital-outpatient-versus-doctor-office-cost-for-physician-administered-cancer-drugs.pdf.
Fitch K, Pyenson B Site of Care Cost Differenes for Medicare Patients Receiving Chemotherapy. Milliman; 2011. Accessed April 23, 2018. http://us.milliman.com/uploadedFiles/insight/health-published/site-of-service-cost-differences.pdf
Lipitz-Snyderman A, Atoria CL, Schleicher SM, Bach PB, Panageas KS. Practice patterns for older adult patients with advanced cancer: physician office versus hospital outpatient setting. J Oncol Pr. 2019;15:e30–8. https://doi.org/10.1200/JOP.18.00315.
Mark M, Thurlimann B, Ribi K, Schar C, Dietrich D, Cathomas R. et al. Patterns of care for patients with metastatic bone disease in solid tumors: A cross-sectional study from Switzerland (SAKK 95/16). J Bone Oncol. 2020;21:100273. https://doi.org/10.1016/j.jbo.2019.100273.
Stoffel ST, von Moos R, Thürlimann B, Cathomas R, Gillessen S, Zürrer-Härdi U. et al. Patterns of care and economic consequences of using bone-targeted agents for castration-sensitive prostate cancer patients with bone metastases to prevent skeletal-related events in Switzerland - the SAKK 95/16 prostate study. Swiss Med Wkly. 2021;151:w20464. https://doi.org/10.4414/smw.2021.20464.
Stopeck A, Rader M, Henry D, Danese M, Halperin M, Cong Z. et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. J Med Econ. 2012;15:712–23. https://doi.org/10.3111/13696998.2012.675380.
Carter JA, Botteman MF. Health-economic review of zoledronic acid for the management of skeletal-related events in bone-metastatic prostate cancer. Expert Rev Pharmacoecon Outcomes Res. 2012;12:425–37. https://doi.org/10.1586/erp.12.31.
Lothgren M, Bracco A, Lucius B, Northridge K, Halperin M, Macarios D. et al. PCN116 cost-effectiveness of denosumab versus zoledronic acid (ZA) for the prevention of skeletal-related events (SRE) in patients with bone metastases from solid tumors in the Netherlands. Value Health. 2011;14:A455 https://doi.org/10.1016/j.jval.2011.08.1217.
Gupta A, Wang P, Ali SA. Use of bone-modifying agents among medicare beneficiaries with multiple myeloma. JAMA Oncol. Published online December 12, 2019. https://doi.org/10.1001/jamaoncol.2019.5426
Sathiakumar N, Delzell E, Yun H, Jooste R, Godby K, Falkson C. et al. Accuracy of medicare claim–based algorithm to detect breast, prostate, or lung cancer bone metastases. Med Care. 2017;55:e144–9. https://doi.org/10.1097/MLR.0000000000000539.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health [grant numbers 1R03CA259863-01 to A.P.M, and P30CA008748]. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
APM: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, visualization, writing-final draft, and writing- review and editing. AMM: data curation, formal analysis, investigation, methodology, resources, software, validation, visualization, and writing-review and editing. KSP: Data curation, formal analysis, investigation, methodology, and writing-review and editing. AS: conceptualization, investigation, methodology, and writing-review and editing. AF: conceptualization, investigation, and writing-review and editing. MM: conceptualization, supervision, and writing-review and editing. The final manuscript was approval by all authors. Dr. Mitchell had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Prior Presentation: American Society of Clinical Oncology Genitourinary Symposium, 2022
Corresponding author
Ethics declarations
Competing interests
APM and AS have no potential conflicts of interest to disclose. AMM declares stock ownership in Johnson & Johnson, DNA, and Teladoc Health. KP declares stock ownership in T2 Biosystems, Adicet Bio, Inc., Chinook Therapeutics, Codexis, and Vincerx Pharma. AF declares consulting for Amgen. MM declares consulting and/or advisory arrangements with Bayer, Novartis, Advanced Accelerator Applications, ORIC Pharmaceuticals, Johnson & Johnson, Curium Pharma, Athenex, Exelixis, AstraZeneca, and Amgen, paid travel for Endocyte and Fujifilm, and research funding from Bayer, Sanofi, Endocyte, Progenics, Corcept Therapeutics, Roche/Genentech, and Janssen.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mitchell, A.P., Meza, A.M., Panageas, K.S. et al. Real-world use of bone modifying agents in metastatic, castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 26, 126–132 (2023). https://doi.org/10.1038/s41391-022-00573-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-022-00573-y