Abstract
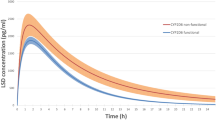
Citalopram is commonly prescribed to patients suffering from major depressive disorder. Some of them do not respond adequately to therapy with citalopram, while many of them experience type A adverse drug reactions. Current research revealed that CYP2C19 isoenzyme is involved in the biotransformation of citalopram. The objective of our study was to investigate the impact of 681G>A polymorphism of the CYP2C19 gene on the efficacy, safety and the concentration/dose indicator of citalopram. Our study enrolled 130 patients with major depressive disorder and comorbid alcohol use disorder (average age–38.7 ± 14.1 years). Therapy regimen included citalopram in an average daily dose of 31.1 ± 14.4 mg per week. Therapy efficacy and safety were evaluated using the international psychometric scales. For genotyping, we performed the real-time polymerase chain reaction. Our findings revealed the statistically significant results in terms of the treatment efficacy evaluation (HAMD scores at the end of the treatment course): (GG) 8.0 [8.0; 9.0] and (GA) 10.0 [9.0; 11.0], p < 0.001. In the safety profile (the UKU scores), the statistical significance was also obtained: (GG) 3.0 [3.0; 4.0] and (GA) 5.0 [4.0; 5.0], p < 0.001. We revealed a statistical significance for concentration/dose indicator of citalopram in patients with different genotypes: (GG) 2.543 [1.659; 4.239] and (GA) 4.196 [2.643; 5.753], p < 0.001). The effect of CYP2C19 genetic polymorphism on the efficacy and safety profiles of citalopram was demonstrated in a group of 130 patients with major depressive disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Boschloo L, Vogelzangs N, Smit JH, van den Brink W, Veltman DJ, Beekman AT, et al. Comorbidity and risk indicators for alcohol use disorders among persons with anxiety and/or depressive disorders: Findings from the Netherlands Study of Depression and Anxiety (NESDA). J Affect Disord. 2011;131:233–42. https://doi.org/10.1016/j.jad.2010.12.014.
Shiv G, Akhilesh J, Manaswi G. Guidelines for the pharmacological management of depression. Indian J Psychiatry. 2017;59:34–50. https://doi.org/10.4103/0019-5545.196973.
Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med. 2001;7:201–4. https://doi.org/10.1016/s1471-4914(01)01986-4.
Aldrich SL, Poweleit EA, Prows CA, Martin LJ, Strawn JR, Ramsey LB. Influence of CYP2C19 metabolizer status on escitalopram/citalopram tolerability and response in youth with anxiety and depressive disorders. Front Pharmacol 2019;10:99. https://doi.org/10.3389/fphar.2019.00099.
Dorji PW, Tshering G. Na-Bangchang KCYP2C9, CYP2C19, CYP2D6 and CYP3A5 polymorphisms in South-East and East Asian populations: a systematic review. J Clin Pharm Ther. 2019;44:508–24. https://doi.org/10.1111/jcpt.12835.
Huang B, Cui DJ, Ren Y, Han B, Yang DP, Zhao X. Effect of cytochrome P450 2C19*17 allelic variant on cardiovascular and cerebrovascular outcomes in clopidogrel-treated patients: a systematic review and meta-analysis. J Res Med Sci. 2017;22:109. https://doi.org/10.4103/jrms.JRMS_590_16. Published 2017 Sep 26.
Moriyama B, Obeng AO, Barbarino J, Penzak SR, Henning SA, Scott SA, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C19 and Voriconazole Therapy [published correction appears in Clin Pharmacol Ther. Clin Pharmacol Ther. 2017;102:45–51. https://doi.org/10.1002/cpt.583.
Mrazek DA, Biernacka JM, O’Kane DJ, Black JL, Cunningham JM, Drews MS, et al. CYP2C19 variation and citalopram response. Pharmacogenet Genomics. 2011;21:1–9. https://doi.org/10.1097/fpc.0b013e328340bc5a.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100.
Zastrozhin MS, Skryabin VY, Torrado M, Petrovna A, Sorokin AS, Grishina EA, et al. Effects of CYP2C19*2 polymorphisms on the efficacy and safety of phenazepam in patients with anxiety disorder and comorbid alcohol use disorder. Pharmacogenomics. 2020;21:111–23. https://doi.org/10.2217/pgs-2019-0019.
Zastrozhin MS, Sorokin AS, Agibalova TV, Grishina EA, Antonenko AР, Rozochkin IN, et al. Using a personalized clinical decision support system for bromdihydrochlorphenylbenzodiazepine dosing in patients with anxiety disorders based on the pharmacogenomic markers. Hum Psychopharmacol. 2018;33:e2677. https://doi.org/10.1002/hup.2677. Epub 2018 Oct 25.
Skryabin VY, Zastrozhin MS, Torrado MV, Grishina EA, Ryzhikova KA, Shipitsyn VV, et al. How do CYP2C19*2 and CYP2C19*17 genetic polymorphisms affect the efficacy and safety of diazepam in patients with alcohol withdrawal syndrome? Drug Metab Pers Ther. 2020. https://doi.org/10.1515/dmpt-2019-0026.
Funding
This work was financially supported by the project 16-15-00227 entitled “Fundamental research and exploratory research in priority areas of research” of the Russian Science Foundation. This work was supported by the grant of the Russian Science Foundation (project No. 18-75-10073).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zastrozhin, M.S., Skryabin, V.Y., Petukhov, A.E. et al. Effects of CYP2C19 genetic polymorphism on the steady-state concentration of citalopram in patients with major depressive disorder. Pharmacogenomics J 21, 435–439 (2021). https://doi.org/10.1038/s41397-021-00219-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41397-021-00219-7