Abstract
Cortical microstructure is influenced by circadian rhythm and sleep deprivation, yet the precise underpinnings of these effects remain unclear. The ratio between T1-weighted and T2-weighted magnetic resonance images (T1w/T2w ratio) has been linked to myelin levels and dendrite density and may offer novel insight into the intracortical microstructure of the sleep deprived brain. Here, we examined intracortical T1w/T2w ratio in 41 healthy young adults (26 women) before and after 32 h of either sleep deprivation (n = 18) or a normal sleep-wake cycle (n = 23). Linear models revealed significant group differences in T1w/T2w ratio change after 32 h in four clusters, including bilateral effects in the insular, cingulate, and superior temporal cortices, comprising regions involved in attentional, auditory and pain processing. Across clusters, the sleep deprived group showed an increased T1w/T2w ratio, while the normal sleep-wake group exhibited a reduced ratio. These changes were not explained by in-scanner head movement, and 95% of the effects across clusters remained significant after adjusting for cortical thickness and hydration. Compared with a normal sleep-wake cycle, 32 h of sleep deprivation yields intracortical T1w/T2w ratio increases. While the intracortical changes detected by this study could reflect alterations in myelin or dendritic density, or both, histological analyses are needed to clarify the precise underlying cortical processes.
Similar content being viewed by others
Introduction
Insufficient sleep causes deficits in cognitive and affective processing [1], and is frequently reported among patients with neurodegenerative and psychotic disorders [2]. Chronic sleep deprivation is also common in mood and anxiety disorders [3]. Paradoxically, acute sleep deprivation has an intriguing antidepressant effect in some patients, peaking after around 32 h [4, 5]. Findings from rodent studies suggest that acute sleep deprivation is linked to structural alterations in the cerebral cortex involving myelin and dendritic spines [6, 7]. However, we lack a clear understanding of the cortical mechanisms underlying sleep deprivation effects in humans.
Using magnetic resonance imaging (MRI) derived indices, several human studies have reported cortical changes following acute sleep deprivation [8, 9]. One night without sleep was linked to volume decreases in the insula and parietal cortex [8], and decreased cortical thickness in the medial parietal cortex [9]. However, the interpretation of the underlying mechanisms is limited by the macrostructural nature of these measures. Using microstructural imaging such as diffusion-weighted imaging (DWI), studies have reported sleep deprivation effects on white matter pathways [10, 11]. This includes alterations in radial diffusivity (RD), which has been related to myelin integrity [12]. DWI has also been applied to measure sleep deprivation effects in the cortex, showing declines in cortical mean diffusivity (MD) [13]. However, the neurobiological correlates of MD changes in the cerebral cortex are complex and not well understood.
Another index of intracortical microstructure is the ratio between T1-weighted and T2-weighted images (T1w/T2w ratio) [14]. Regional variation in T1w/T2w ratio has been found to relate to variation in histologically derived myelin levels [14, 15], and, recently, also to dendrite density [16]. While awaiting further histological comparisons, the T1w/T2w ratio might be sensitive to the very intracortical properties reported in rodents following sleep deprivation. Interestingly, a recent cross-sectional study reported associations between T1w/T2w ratio and self-reported sleep quality and sleep duration in several cortical regions [17]. While correlational, these findings may suggest that the T1w/T2w ratio is sensitive to sleep-related processes. Hence, testing whether acute sleep deprivation leads to alterations in the T1w/T2w ratio might help illuminate the underlying substrates of sleep deprivation effects in humans.
In this study, we investigated the effects of 32 h of wake and sleep on cortical microstructure using the T1w/T2w ratio. In total, 41 healthy young adults underwent MRI before and after either sleep deprivation (n = 18) or a normal sleep-wake cycle (NSW, n = 23). As recent studies report time-of-day effects on other MRI modalities [9, 10, 13, 18,19,20,21,22,23,24], we aimed to control for such effects by comparing the effect of 32 h of sleep deprivation with NSW. To this end, we first estimated within-subjects changes between the two MRI scans and then assessed whether these were significantly different between the sleep deprivation group and the NSW group. Based on (i) the links between sleep deprivation and myelin and dendritic spine alterations and (ii) the sensitivity of the T1w/T2w ratio to the latter microstructural processes, we hypothesised that the two groups would show significantly different changes in the T1w/T2w ratio. To reduce the potential for confounding factors, we monitored a number of Zeitgeber signals, such as food intake, caffeine intake, physical activity and exposure to blue-emitting light. To assess potential functional correlates of cortical changes, we also explored associations between the T1w/T2w ratio and sleepiness and lapses in attention.
Methods and materials
Ethics statement
This study was approved by the Regional Committee for Medical and Health Research Ethics, South-Eastern Norway (REK Sør-Øst, ref: 2017/2200) and conducted in line with the Declaration of Helsinki of 2013. All participants gave their written informed consent prior to participation and received NOK 1000.
Participants
The recruitment procedure was described in detail previously [18]. Volunteers were recruited through social media and a national newspaper advert. 127 volunteers underwent clinical screening over the phone and 41 were excluded due to meeting one of the following exclusion criteria: history or presence of any psychiatric disorder by means of screening questions from MINI neuropsychiatric inventory [25]; severe or chronic somatic disorder by a slightly modified version of Stanley Foundation Entry Questionnaire, which covers 21 somatic including neurological disorders and history of head injury [26]; current intake of any regular medication; smoking; caontraindications to MRI or living more than one hour of travel away from the MRI facility. Of the remaining 86 volunteers, 15 withdrew their participation, and 22 were cancelled due to logistic reasons. After study start, one participant was excluded due to illness and two due to claustrophobic reaction in the scanner. An additional five were excluded due to incomplete T2w data. The final sample consisted of 41 healthy adults (mean age 26 ± 6.9 years; age range 18 to 46 years; 26 women).
Study design
Figure 1 presents an overview of the study design. Due to time constraints and to reduce the strain on participants, T2-weighted MRI required for T1w/T2w ratio estimations were obtained only at the first and last MRI scan. Participants underwent the first MRI scan at time point 1 (TP1) in the morning of the first day at Oslo University Hospital, Rikshospitalet. They arrived fasting after a night of regular sleep in their own home (around 9 AM). After spending the day at the hospital, participants completed their second MRI scan (around 8 PM) and were randomised by draw to either go home to sleep (NSW group) or to stay awake at the hospital during the night (sleep deprivation group). In the morning, the NSW group returned, and both groups underwent their third MRI scan (around 8 AM). They then spent a second day at the hospital. The final scan took place in the afternoon after approximately 32 h since study start (around 4 PM, TP2).
They also underwent MRI (not including T1w- and T2w scans) in the evening on the first day and in the morning on the second day. During the study, participants underwent tests of subjective sleepiness (Karolinska Sleepiness Scale, KSS) and objective alertness (Psychomotor Vigilance Task, PVT) every second and third hour, respectively. Seven days prior to the first MRI scan, participants underwent measurements of sleep habits by actigraphy and self-report sleep diary.
A blood sample was obtained from each participant after each scan for analysis of haematocrit, to estimate hydration level. Participants followed a standardised activity plan in the company of a research assistant (see Voldsbekk et al., 2020 for a detailed description). To ensure that no one fell asleep during the MRI scans, a camera (Model 12M-i, MRC Systems GmbH, Heidelberg, Germany) inside the scanner bore was used to monitor the eyes of the participants.
Assessment of sleep habits
Seven days prior to study start, participants recorded their sleep pattern by self-report and actigraphy. Participants in the NSW group also recorded their sleep pattern during the night of the study. Self-report data was recorded each day by a 10-item semi-structured sleep diary [27], which assessed sleep-related behaviour and quality. The scale was modified to also include two items on caffeine intake and nicotine intake. Actigraphy data was recorded by a Condor Instruments ActTrust actigraph (São Paulo, Brazil), which measured an individual’s movements by a digital tri-axial accelerometer with a 60 s epoch. In addition, participants completed five standardised questionnaires regarding their sleep habits: the Bergen Insomnia Scale [28], the Epworth Sleepiness Scale [29], the Pittsburgh Sleep Quality Index [30] and the Horne-Østberg Morningness Eveningness Questionnaire [31]. These questionnaires measure insomnia-related symptoms, daytime sleepiness, sleep quality, and chronotype, respectively.
Assessment of acute sleepiness and alertness
After each scan and every other hour throughout the study, participants completed the Karolinska Sleepiness Scale (KSS), which is a 1-item self-report rating of sleepiness on a nine-point Likert scale [32]. To measure objective alertness, participants completed a computerised psychomotor vigilance task (PC-PVT) [33] after each scan and every third hour of the study. For ten minutes, a five-digit millisecond (ms) counter was presented as the visual stimulus on a screen with random intervals. Participants clicked a mouse button in response. Performance was quantified as number of minor attentional lapses, i.e. reaction times (RT) > 500 ms, which has been found to be a more sensitive measure of sleep deprivation-related alertness compared to mean or median RT [34]. The task was run using Matlab 2017a (MathWorks, Massachusetts, USA) on a Lenovo laptop V510-15IKB with Windows 10 Pro and a Cooler Master mouse model SGM-1006-KSOA1. The laptop display had a refresh rate of 60 Hz.
MRI acquisition
Imaging was performed on a 3 T Siemens Magnetom Prisma scanner (Siemens Healthcare, Erlangen, Germany) using a 32-channel head coil. The scan protocol consisted of a four-echo T1w multi-echo magnetisation-prepared rapid gradient-echo sequence (repetition time/echo times = 2530 ms/1.69-3.55-5.41-7.27 ms, field-of-view = 256 x 256mm2, voxel size = 1.0 × 1.0 × 1.0mm3, flip angle = 7°, acceleration factor = 2, acquisition time = 6 min 3 seconds) and a bandwidth-matched T2w sequence (repetition time/echo time = 3200 ms/563 ms, field-of-view = 256 x 256mm2, voxel size = 1.0 × 1.0 × 1.0mm3, acceleration factor = 2, acquisition time = 4 min 24 seconds). The four T1w images were used to estimate a root mean square image which was submitted to further analyses [35].
MRI preprocessing
T1w/T2w maps were created using the Human Connectome Project (HCP) processing pipeline (https://github.com/Washington-University/Pipelines) [36], including processing with FreeSurfer version 5.3 (http://surfer.nmr.mgh.harvard.edu), similarly to the processing done by Grydeland and colleagues [37]. The T1w volume was divided by the aligned [38] and spline interpolated T2w volume, yielding a T1w/T2w ratio volume. For regional T1w/T2w maps, a multimodal parcellation was utilised which divided each cerebral hemisphere into 180 regions [39]. T1w/T2w values were sampled from the white matter/grey matter boundary, at three cortical depths around the middle of the cortical mantle (30%, 50%, and 70% into the cortex).
Statistical analyses
In order to ensure homogeneity of the sample, two-sample t-tests of group differences were run for each demographic and sleep-wake characteristic. In addition, we ran a paired t-test across groups to ensure that participants had a normal sleep routine at the outset of the experiment (i.e. a comparison of their sleep duration the night before the study with their average sleep duration for the past week). To ensure no group differences due to random sampling, we also ran a mixed two-way analysis of variance (ANOVA) testing whether there were any group differences in how participants slept the night before the study compared with the previous week. To test for group differences in the T1w/T2w ratio between TP1 and TP2, the symmetrised percentage change (SPC) was calculated, which has been shown to be more robust than percentage change [40]:
Group comparison was then run using linear regression models in R [41] in each of the 360 atlas regions. To reduce potential partial volume effects [42], the main analysis was run using the T1w/T2w ratio measured at a 50% depth. As a sensitivity analysis to assess the potential effects of cortical depth, we then reran the analysis using the T1w/T2w ratio from 30% and 70% into the cortex. As another sensitivity analysis, we re-ran our main analysis of group differences in the changes over time, using the difference between time points as outcome metric (T1w/T2w at TP 2 - T1w/T2w at TP1). The Euler number, which has been shown to be a useful index of head movement in young adults [43], was extracted from each T1w volume and included as a covariate. To control for multiple comparisons, we ran a custom permutation-based cluster size correction analysis across ROIs in R. A cluster was defined as adjacent regions, i.e. regions sharing a border, showing p-values below the cluster-forming threshold of p = 0.05. This threshold was chosen to maximise sensitivity in the smaller and less noisy parcellated brain map (compared to a voxel-wise map). To build a null model, we then ran 5000 permutations repeating the group analysis, re-shuffling the group membership for each run, and counting the maximum cluster size. Then, the size of the actual clusters was compared with this distribution of 5000 maximum cluster size from random group orderings. As a measure of effect size we calculated Hedge’s g [44] of the group difference in mean change over time using the effectsize packages in R [45]. For simplicity of interpretation, as there was no difference in head movement between groups, this effect measure was calculated without head movement as a covariate (in contrast to the actual analysis).
For significant clusters, we then extracted the mean T1w/T2w ratio to perform sensitivity analyses. First, we assessed whether the group effect remained significant when including mean cortical thickness across the cluster, and haematocrit, an index of hydration. Second, to explore the functional relevance of changes in T1w/T2w ratio from TP1 to TP2, interaction analyses were performed between changes in T1w/T2w ratio and changes in sleepiness and alertness, as measured by KSS and minor lapses on the PC-PVT. To examine whether our choice of vigilance measure (minor lapses) influenced the results, we re-ran parts of the analysis using alternative measures of vigilance, namely median reaction time (RT) and RT variability (defined as the variance in RT). To correct for the multiple tests, the resulting p-values were adjusted by applying false discovery rate correction (FDR) [46]. We also utilised neurosynth.org, a data-driven tool for meta-analysis of the large primary literature on task-related functional MRI [47], to identify functions most implicated in the observed changes. The top 25 terms were extracted as a word cloud in which the size and colour saturation of the words correspond to the frequencies associated with each term.
Results
Sleep pattern assessment
As seen in Table 1, there were no differences in demographics or sleep-wake characteristics between groups. All participants had slept approximately seven hours each night for the past week, as recorded by self-report and corroborated by actigraphy measurements. For the NSW group, there was no significant difference in total sleep time on the night between the first and second day of the study compared to the night prior to study start (t(12) = −0.18, p = 0.86). Across groups, participants slept significantly shorter on the night before the study compared to their weekly average (t(35) = 3.55, p = 0.001). When comparing the night before the study to the weekly average between groups, we found a main effect of sleep duration (F(1,34) = 12.22, p = 0.001), but no effect for group (F(1,34) = 1.44, p = .24) and no interaction effect (F(1,34) = 0.02, p = 0.89). Hence, the random sampling resulted in groups that had similar sleep duration before the start of the study.
Effect of 32 h of sleep deprivation and normal sleep-wake on cortical microstructure
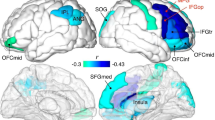
The change from TP1 to TP2 did not differ between groups for hydration or in-scanner head movement (p = 0.01 and p = 0.57, respectively, see Fig. S1). As shown in Fig. 2A, B, the group comparisons revealed four cortical clusters exhibiting significant differences in T1w/T2w ratio change from TP1 to TP2, spanning 37% of the ROIs in the right hemisphere (RH) and 16% in the left hemisphere (LH). Specifically, across clusters, the sleep deprived group showed an increase in T1w/T2w ratio, while the NSW group showed a decrease. In the RH, there were 66 regions across two clusters (61 and 5 regions, df = 38, p < 0.0001 and p = 0.004, Hedge’s g = 0.83 and 0.74, respectively) in predominantly the insular, cingulate, parietal, and superior temporal cortices, while in the LH there were 28 regions across two clusters (13 and 15 regions, df = 38, p < 0.0001 for both, Hedge’s g = 0.81 and 0.77, respectively) predominantly in the insular, cingulate, superior temporal and medial frontal cortices. These results indicate that the within-subject change between the two MRI scans were significantly different between the sleep deprivation group and the NSW group.
A Surface maps of group differences (p-values). Displayed are the regions of the four clusters showing significant group differences in symmetrised percentage change (SPC) in T1w/T2w ratio between the sleep deprivation group and NSW group in the right hemisphere (RH, left side) and the left hemisphere (LH, right side). Each cluster was p < 0.05, and the p-values for each region (before cluster-correction) are presented for illustration. B Information for each of the 4 clusters (C1-C4). Bi Violin-box-scatter plots for the mean T1w/T2w ratio for participants in each group. Bii Surface maps of each cluster separately (p-values). Biii The distribution of maximum cluster sizes across random groupings (grey) with the actual cluster size for each cluster identified with the triangle (magenta).
The first sensitivity analysis showed that the group difference in each cortical cluster remained significant when controlling for hydration and cortical thickness (p < 0.05), except for the smallest cluster with the initially lowest effect (5-region RH cluster, p = 0.55). To assess whether the results were unduly influenced by one participant in the sleep deprivation group showing high cluster values in three of four clusters (see Fig. 2Bi), we excluded this person was and reran the group comparison. As shown in Fig. S2, the results were similar to the main analysis, with four clusters, comprising 77 and 14 regions in RH and LH, respectively, showing group differences. The Hedge’s g in the RH clusters were −0.96 and −0.70, while in the LH −0.83 and −0.84, respectively.
When using a raw difference score as the outcome metric (T1w/T2w at TP 2 - T1w/T2w at TP1), two clusters showed significant differences in change over time between groups, comprising a total of 64 regions (36%) in the RH, and two clusters comprising a total of 33 regions (18%) in the LH. When excluding the one potential outlier, the corresponding results showed two clusters of 78 regions (43%) in the RH, and three clusters of 20 regions (11%) in the LH.
As shown in Fig. 3, there was regional variation in the strength of the effect specific to each group. Specifically, the NSW group showed a stronger reduction across medial parietal regions, fusiform gyrus, and the posterior cingulate cortex, while the sleep deprived group showed a stronger increase in the anterior cingulate cortex, as well as both lateral and medial frontal regions. As determined by neurosynth.org [47] (Fig. 4), the observed changes implicated regions most strongly involved in attention, listening, movement and pain.
To assess whether the higher number of RH regions showing group differences was partly due to a thresholding effect, we summarised the p-values of the regions in the LH which were only significant in the RH. Of these 38 LH regions, the median p-value was 0.10 (minimum – maximum is 0.02 – 0.55), indicating that a portion of the LH regions likely would have been significant with higher statistical power.
The sensitivity analyses assessing the effects of cortical depth, shown in Fig. 5, revealed significant group differences using T1w/T2w ratio values extracted from 30% and 70% cortical depth. At 30% depth, a slightly higher number of regions significantly differed between groups (97 RH regions and 29 LH regions), as compared with the results from 50% depth, while a slightly lower number of significant regions were found at 70% depth (47 RH regions and 14 LH regions).
Maps showing significant group differences in T1w/T2w ratio in the right hemisphere (RH, left side) and left hemisphere (LH, right side) for 30%, 50% and 70% cortical depth. Top row. T1w/T2w ratio sampled from 30% into the cortex from the white/grey matter boundary. Middle row. 50% cortical depth (as shown in Fig. 2A). Bottom row. 70% cortical depth.
Associations between changes in cortical microstructure and sleepiness and attention
The difference in subjective sleepiness and in objectively measured attentional lapses over the 32 h are presented in Fig. S3, and the distribution of change values in Fig. S4. The change in subjective sleepiness differed between the groups (see Table 1), with the sleep deprived group showing increased sleepiness (higher KSS) at TP2. There was no group difference in change in lapses in attention. Alternative measures of attention from the PVT, namely median RT and the variance of RT, showed similar group differences in alertness (see Fig. S5).
The interaction analyses of an association between T1w/T2w ratio changes and sleepiness and attentional lapses, did not show any significant results after FDR-correction (p > 0.28, these analyses were run on the results excluding the participant with a large change value).
The uncorrected results showed a relation between changes in T1w/T2w ratio and sleepiness (see Fig. S6), with an effect in the 9-region LH cluster (p = 0.04), while the other clusters did not show effects (p = 0.61 for RH cluster 1, p = 0.56 for RH cluster 2, and p = 0.25 for LH cluster 2). The effect was due to a negative correlation between T1w/T2w change and sleepiness change in the sleep deprived group (r = −0.7, p = 0.003), while the NSW group showed an opposite, and weaker relation (r = 0.27, p = 0.27). Thus, in the sleep deprivation group, a stronger increase in T1w/T2w ratio was related to lower subjective sleepiness.
The uncorrected results of changes in T1w/T2w ratio and lapses in attention (see Fig. S7) showed an interaction in the 4-region RH cluster (p = 0.022), while the other clusters did not show effects (p = 0.43 for RH cluster 1, p = 0.43 for LH cluster 1, and p = 0.36 for LH cluster 2). This trend was due to a positive correlation between T1w/T2w change and alertness change in the NSW group (r = 0.67, p = 0.012), while the sleep deprivation group showed a weaker relation (r = −0.16, p = 0.54). The results using alternative measures of vigilance were similar, with only the the 4-region RH cluster showing significant (variance RT p = 0.01) or near-significant results (median RT p = 0.059).
Discussion
The findings of the present study indicate that 32 h of sleep deprivation yields different intracortical changes in the T1w/T2w ratio than a NSW cycle. The differences between the sleep deprivation and the NSW groups were more prominent in the RH, but were observed in both hemispheres in insular, cingulate and superior temporal cortices. This includes regions involved in attentional, auditory, movement and pain processing [47]. Across regions, the sleep deprived group showed an increased T1w/T2w ratio, whereas the NSW group exhibited a reduced ratio. The effects were not explained by an estimate of in-scanner head movement, were present at various depths in the cortex, and 95% of the effects remained significant after adjusting for cortical thickness and hydration. Although speculative, candidate neurobiological mechanisms of the observed cortical effects include myelin and dendrite density, previously linked to the T1w/T2w ratio [14,15,16].
We found group differences in T1w/T2w ratio change in the bilateral cingulate, insula, and superior temporal cortices and in right parietal and left middle frontal regions. Although this is the first study of T1w/T2w ratio in sleep deprivation, these results overlap with findings from three previous studies. The first study reported reduced cortical volume mainly in the insular, parietal, posterior cingulate, motor, and somatosensory cortices after 36 h of sleep deprivation [8]. The second study found increased grey matter density in the frontal pole and the superior and middle frontal gyri, as well as decreased volume and thickness in the temporal pole after 24 h of sleep deprivation [48]. The third study reported reduced thickness in bilateral medial parietal regions, yet did not detect a significant group by time interaction effect when compared to a NSW group [9]. Thus, the results of the present and previous studies suggest that acute sleep deprivation is associated with cortical alterations, mainly in frontal, temporal, parietal, and insular cortices. The current study points to new effects in the bilateral anterior cingulate, the superior temporal (including auditory cortex), and the left medial frontal regions. This difference in results may reflect the differences in study designs, but also indicate that the microstructural T1w/T2w ratio has greater sensitivity to the intracortical effects of wake and sleep deprivation.
We observed increased T1w/T2w ratio in the sleep deprived group. Further histological studies are needed to elucidate the neurobiology underlying these T1w/T2w ratio changes, yet they may be linked to wake-related processes, circadian rhythm mechanisms, or a combination of the two [49]. Although the precise neural effects of wake and sleep remain to be clarified, the synaptic homeostasis hypothesis posits that wake and sleep are associated with net increases and decreases, respectively, in densities of synapses and dendrites [50]. Consistent with this hypothesis, spontaneous and enforced wake in animals were linked to increased dendritic branching and synapse number [6, 51, 52] and reduced synapse pruning [53]. Thus, although speculative, T1w/T2w ratio changes within the sleep deprivation group of the present study could be related to wake-induced increases in synaptic and dendritic densities.
An alternative explanation for the increased T1w/T2w ratio after sleep deprivation is cortical myelin changes. The T1w/T2w ratio correlates with cortical myelin content [14], and the ratio was higher in myelinated than demyelinated cortex in multiple sclerosis [15]. There is a scarcity of studies of cortical myelin after sleep deprivation in humans, yet two previous MRI studies reported RD reductions in human white matter after extended wake [10, 18]. Reduced RD is, in general, associated with increased myelination [12]. Furthermore, a growing body of animal data links sleep and wake to myelin and oligodendrocyte alterations [7, 54]. Thus, further studies should clarify whether changes to myelin and oligodendrocytes contribute to the T1w/T2w ratio increases found in the current study.
We observed a T1w/T2w ratio reduction within the NSW group from the morning of the first study day to the afternoon of the second day. Several lines of evidence point to sleep-wake as an active process throughout both night and day, and potentially involving alterations in brain microstructure. Two separable, yet interacting processes are considered to regulate sleep-wake: sleep homeostasis, by which increasing sleep pressure accumulates as a function of time spent awake, and the circadian rhythm, which is an intrinsic oscillating cycle of 24 h regulated by exposure to daylight [49]. In line with this framework, diurnal fluctuation has been observed in the transcription of genes related to macromolecule homeostasis [55, 56], oligodendrocyte proliferation, phospholipid synthesis and myelination [7]. As such, we speculate that T1w/T2w changes from morning to evening of the first study day would have been reversed by the night of sleep, and that the NSW group changes detected here reflect morning- to-afternoon processes of the second study day. Importantly, these hypotheses must be tested in future studies since the current work obtained T2-weighted MRI only at baseline (TP1) and after 32 h (TP2). Notwithstanding this limitation, the T1w/T2w ratio reduction from the morning of the first study day to the afternoon of the second day within the NSW group is consistent with recent studies reporting time-of-day effects on other MRI modalities [9, 10, 13, 18,19,20,21,22,23,24].
Taken together, the directionality of the T1w/T2w ratio changes in the two groups were, interestingly, in the opposite direction. Simultaneous MRI and histological analyses are required to clarify whether regular wake length, e.g., from morning to the same afternoon, could be associated with neural processes that qualitatively differ from those induced by sleep deprivation. The relationships between wake length and cortical alterations are not necessarily linear. In support of this notion, one study observed decreases in cortical thickness from morning to afternoon (2 h) [21], whereas another study detected increased cortical thickness from morning to evening (14 h) [9]. Furthermore, the T1w/T2w ratio changes observed in the current study could also reflect the likely complex and dynamic relationship between wake-induced neural processes and the circadian rhythm of the cortex.
The T1w/T2w ratio group differences comprised regions involved in attentional processes, which are amongst the first to deteriorate in response to sleep deprivation [57]. However, we found no significant relationship between attentional lapses and T1w/T2w ratio. Previous studies reported significant associations between sleepiness and changes in cortical thickness [9] and cortical density [48] after sleep deprivation. Consistent with these results, we found a weak association between reported sleepiness and T1w/T2w ratio change in one of the smaller clusters, but this did not survive FDR-correction. Thus, while the group differences suggest significant effects of sleep deprivation, the functional consequences of the intracortical T1w/T2w ratio changes among the sleep deprived individuals remain to be elucidated.
Several study limitations should be noted. First, participants had slept less the night before the study than their weekly average. However, if we assume that sleep reverses microstructural changes after wakefulness, we expect less sleep to result in attenuation, rather than inflation, of any sleep deprivation-related changes. Second, participants in the NSW group went home to sleep, which was measured with an actigraph and a sleep diary. Sleep in a laboratory with polysomnography would have resulted in greater control of exposure to Zeitgebers and sleep length. Third, the study did not include T2w MRI acquisitions from the evening of the first study day and the next morning, which limited our ability to interpret the temporal dynamics of the T1w/T2w ratio changes. Finally, the sample size was modest, and the analyses indicated that more LH effects would have been significant with higher statistical power. The lateralisation of T1w/T2w ratio effects with more significant clusters in the RH should therefore be cautiously considered. In addition, the between-subject design employed introduces additional degrees of uncertainty related to possible inter-subject differences when it comes to vulnerability to sleep deprivation. Given the relatively modest sample size, this represents an important consideration in the interpretation of our results.
The current study provides evidence that compared with a NSW cycle, 32 h of sleep deprivation yields intracortical microstructural changes as indexed by the T1w/T2w ratio. These effects were observed within regions linked to attentional, auditory, movement and pain processing and were not explained by an estimate of in-scanner head movement, minimally affected by cortical thickness, and hydration, and were present at various depths in the cortex. The T1w/T2w ratio changes could reflect alterations in myelin or dendrite density, or both, and histological analyses are needed to clarify the precise underlying cortical processes.
References
Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–29.
Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–99.
Peterson MJ, Benca RM. Sleep in mood disorders. Psychiatr Clin. 2006;29:1009–32.
Boland EM, Rao H, Dinges DF, Smith RV, Goel N, Detre JA, et al. Meta-analysis of the antidepressant effects of acute sleep deprivation. J Clin Psychiatry. 2017;78:e1020–e1034.
Wu C, Bunney E. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psych. 1990;147:14–21.
de Vivo L, Bellesi M, Marhsall W, Bushong EA, Ellisman MH, Tononi G, et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 2017;355:507–10.
Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C, et al. Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci. 2013;33:14288–14300.
Dai XJ, Jiang J, Zhang Z, Nie X, Liu BX, Pei L, et al. Plasticity and susceptibility of brain morphometry alterations to insufficient sleep. Front Psychiatry. 2018;9:266.
Elvsåshagen T, Zak N, Norbom LB, Pedersen P, Quraishi SH, Bjørnerud A, et al. Evidence for cortical structural plasticity in humans after a day of waking and sleep deprivation. NeuroImage. 2017;156:214–23.
Elvsåshagen T, Norbom LB, Pedersen P, Quraishi SH, Bjørnerud A, Malt UF, et al. Widespread changes in white matter microstructure after a day of waking and sleep deprivation. PLoS ONE. 2015;10:1–15.
Voldsbekk I, Groote I, Zak N, Roelfs D, Geier O, Due-Tønnessen P, et al. Sleep and sleep deprivation differentially alter white matter microstructure: A mixed model design utilising advanced diffusion modelling. NeuroImage. 2021;226:117540.
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–36.
Bernardi G, Cecchetti L, Siclari F, Buchmann A, Yu X, Handjaras G, et al. Sleep reverts changes in human gray and white matter caused by wake-dependent training. NeuroImage. 2016;129:367–77.
Glasser MF, Van DC. Essen, mapping human cortical areas <em>in vivo</em> based on myelin content as revealed by t1- and t2-weighted MRI. J Neurosci. 2011;31:11597–616.
Nakamura K, Chen JT, Ontaneda D, Fox RJ, Trapp BD. T1‐/T2‐weighted ratio differs in demyelinated cortex in multiple sclerosis. Ann Neurol. 2017;82:635–9.
Righart R, Biberacher V, Jonkman LE, Klaver R, Schmidt P, Buck D, et al. Cortical pathology in multiple sclerosis detected by the T1/T2-weighted ratio from routine magnetic resonance imaging. Ann Neurol. 2017;82:519–29.
Toschi N, Passamonti L, Bellesi M. Sleep quality relates to emotional reactivity via intracortical myelination. Sleep. 2021;44:zsaa146.
Voldsbekk I, Maximov II, Zak N, Roelfs D, Geier O, Due-Tønnsessen P, et al. Evidence for wakefulness-related changes to extracellular space in human brain white matter from diffusion-weighted MRI. NeuroImage. 2020;212:116682–116682.
Elvsåshagen T, Mutsaerts HJMM, Zak N, Norbom LB, Quraishi SH, Pedersen PØ, et al. Cerebral blood flow changes after a day of wake, sleep, and sleep deprivation. NeuroImage. 2019;186:497–509.
Kaufmann T, Elvsåshagen T, Alnæs D, Zak N, Pedersen P, Norbom LB, et al. The brain functional connectome is robustly altered by lack of sleep. NeuroImage. 2016;127:324–32.
Trefler A, Sadeghi N, Thomas AG, Pierpaoli C, Baker CI, Thomas C. Impact of time-of-day on brain morphometric measures derived from T1-weighted magnetic resonance imaging. NeuroImage. 2016;133:41–52.
Thomas C, Sadeghi N, Nayak A, Trefler A, Sarlls J, Baker CI, et al. Impact of time-of-day on diffusivity measures of brain tissue derived from diffusion tensor imaging. NeuroImage. 2018;173:25–34.
Hodkinson DJ, O’Daly O, Zunzain PA, Pariante CM, Lazurenko V, Zelaya FO, et al. Circadian and homeostatic modulation of functional connectivity and regional cerebral blood flow in humans under normal entrained conditions. J Cereb Blood Flow Metab. 2014;34:1493–9.
Jiang C, Yi L, Su S, Shi C, Long X, Xie G, et al. Diurnal variations in neural activity of healthy human brain decoded with resting-state blood oxygen level dependent fMRI. Front Hum Neurosci. 2016;10:634.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Wellier E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin psychiatry. 1998;59 Suppl 2:22–33.
Suppes T, Leverich GS, Keck PE, Nolen WA, Denicoff KD, Altshuler LL, et al. The stanley foundation bipolar treatment outcome network. II. demographics and illness characteristics of the first 261 patients. J Affect Disord. 2001;67:45–59.
Bjorvatn B. Søvndagbok. SOVno. 2018; https://helse-bergen.no/nasjonal-kompetansetjenestefor-sovnsykdommer-sovno/sovndagbok-sovno.
Pallesen S, Bjorvatn B, Nordhus IH, Sivertsen B, Hjørnevik MARI, Morin CM. A new scale for measuring insomnia: the bergen insomnia scale. Percept Mot Skills. 2008;107:691–706.
Johns MW. A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep. 1991;14:540–5.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, Reynolds CF, et al. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Horne JA, Östberg O. A self assessment questionnaire to determine morningness eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110.
Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37.
Khitrov MY, Laxminarayan S, Thorsley D, Ramakrishnan S, Rajaraman S, Wesensten NJ, et al. PC-PVT: a platform for psychomotor vigilance task testing, analysis, and prediction. Behav Res Methods. 2014;46:140–7.
Basner M, Dinges DF. Maximizing sensitivity of the Psychomotor Vigilance Test (PVT) to sleep loss. Sleep. 2011;34:581–91.
Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–94.
Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013;80:105–24.
Grydeland H, Vértes PE, Váša F, Romero-Garcia R, Whitaker K, Alexander-Bloch AF, et al. Waves of maturation and senescence in micro-structural mri markers of human cortical myelination over the lifespan. Cereb Cortex. 2019;29:1369–81.
Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72.
Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–8.
Berry DA, Ayers GD. Symmetrized percent change for treatment comparisons. Am Statistician. 2006;60:27–31.
R Core Development Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna: 2006. http://www.R-project.org.
Huntenburg JM, Bazin P-L, Margulies DS. Large-scale gradients in human cortical organization. Trends Cogn Sci. 2018;22:21–31.
Rosen AF, Roalf, David R, Ruparel K, Blake J, Seelaus K, Villa LP, et al. Quantitative assessment of structural image quality. Neuroimage. 2018;169:407–18.
Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, Florida: Academic press; 1985.
Ben-Shachar M, Lüdecke D, Makowski D. Effectsize: Estimation of effect size indices and standardized parameters. J Open Source Softw. 2020;5:2815.
Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol). 1995;57:289–300.
Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat methods. 2011;8:665–70.
Sun J, Zhao R, Yang X, Deng H, Zhu Y, Chen Y, et al. Alteration of brain gray matter density after 24 h of sleep deprivation in healthy adults. Front Neurosci. 2020;14:754.
Borbély AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. J Sleep Res. 2016;25:131–43.
Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34.
Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–81.
Donlea JM, Alam MN, Szymusiak R. Neuronal substrates of sleep homeostasis; lessons from flies, rats and mice. Curr Opin Neurobiol. 2017;44:228–35.
Li W, Ma L, Yang G, Gan WB. REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci. 2017;20:427–37.
Bellesi M, Haswell JD, de Vivo L, Marshall W, Roseboom PH, Tononi G, et al. Myelin modifications after chronic sleep loss in adolescent mice. Sleep. 2018;41:zsy034.
Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, et al. Macromolecule biosynthesis: a key function of sleep. Physiolo. Genomics. 2007;31:441–57.
Cirelli C, LaVaute TM, Tononi G. Sleep and wakefulness modulate gene expression in drosophila. J Neurochemistry. 2005;94:1411–9.
Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39.
Acknowledgements
This project was funded by research grants from the Norwegian South-East Health Authorities (2018077, 2017090, 2015078), the Research Council of Norway (249795), the Centre for Digital Life Norway, the Ebbe Frøland foundation, the Norwegian Competence Center for Sleep Disorders, Haukeland University Hospital, Bergen (www.sovno.no), the University of Oslo Life Science summer scholarship for students and a research grant from Mrs. Throne-Holst.
Author information
Authors and Affiliations
Contributions
IG, TE, and AB designed the study. IV, NZ, DR, YSK, L-LL, MS, TYB, and IG collected the data. IV, TE, and HG analysed the data. IG, AB, IIM, OG, PD-T, EB, BB, UFM and LTW contributed with conceptual and methodological input. IV, TE, and HG wrote the first draft of the paper and all authors contributed to the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
TE received speaker’s honoraria from Lundbeck and Janssen Cilag and is a consultant to BrainWaveBank and Synovion. NZ received speaker’s honoraria from Lundbeck.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Voldsbekk, I., Bjørnerud, A., Groote, I. et al. Evidence for widespread alterations in cortical microstructure after 32 h of sleep deprivation. Transl Psychiatry 12, 161 (2022). https://doi.org/10.1038/s41398-022-01909-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-01909-x