Abstract
Age increases the risk for cognitive impairment and is the single major risk factor for Alzheimer’s disease (AD), the most prevalent form of dementia in the elderly. The pathophysiological processes triggered by aging that render the brain vulnerable to dementia involve, at least in part, changes in inflammatory mediators. Here we show that lipoxin A4 (LXA4), a lipid mediator of inflammation resolution known to stimulate endocannabinoid signaling in the brain, is reduced in the aging central nervous system. We demonstrate that genetic suppression of 5-lipoxygenase (5-LOX), the enzyme mediating LXA4 synthesis, promotes learning impairment in mice. Conversely, administration of exogenous LXA4 attenuated cytokine production and memory loss induced by inflammation in mice. We further show that cerebrospinal fluid LXA4 is reduced in patients with dementia and positively associated with cognitive performance, brain-derived neurotrophic factor (BDNF), and AD-linked amyloid-β. Our findings suggest that reduced LXA4 levels may lead to vulnerability to age-related cognitive disorders and that promoting LXA4 signaling may comprise an effective strategy to prevent early cognitive decline in AD.
Similar content being viewed by others
Introduction
Inflammation constitutes an essential defensive mechanism that is dysregulated in aging and in several chronic and neurodegenerative disorders, including Alzheimer’s disease (AD) [1, 2]. Accordingly, a chronic imbalance favoring disproportionate pro-inflammatory responses in the brain and periphery is thought to mediate age-related cognitive decline and AD pathogenesis [3,4,5,6,7,8,9,10,11].
Proper control of inflammatory responses depends on resolution processes, which are controlled by lipid mediators known as resolvins, such as lipoxins [12]. Lipoxin A4 (LXA4) is an eicosanoid derived from arachidonic acid through transcellular metabolic pathways that depend on the enzymatic activity of 5-lipoxygenase (5-LOX) [12,13,14]. In the periphery, LXA4 activates the G-protein-coupled receptor ALX to modulate gene expression towards the resolution of inflammation and to promote immune cell recruitment to the site of infection or damage [12]. Putative effects of LXA4 in the brain have long remained elusive, due to the low expression of ALX receptors in the central nervous system (CNS) [15].
We and others have suggested that LXA4 protects the central nervous system against injuries [16,17,18,19,20], including amyloid-β (Aβ) toxicity in mice [17, 21, 22]. However, while the processes that propagate cytokine-mediated inflammation in AD have been thoroughly investigated, very little is known about the impact and mechanisms of LXA4 on brain function. Given that ALX receptors are poorly expressed in the central nervous system [15], other mechanisms should be responsible for the brain actions of LXA4. We have previously demonstrated that LXA4 binds to CB1 receptors in the brain to stimulate endocannabinoid signaling [21]. These findings raise the prospect that LXA4 might be relevant to complex brain functions and that LXA4 signaling might be impaired in age-related cognitive diseases linked to aberrant inflammation, such as AD.
Herein we report that mammalian neurons and microglia produce considerable levels of LXA4. We further demonstrate that brain and peripheral LXA4, as well as memory, decline with aging and that genetic suppression of 5-LOX results in memory impairment in mice. Conversely, administration of exogenous LXA4 attenuated cytokine production and memory loss induced by an inflammatory stimulus in mice. We finally present results from a human study demonstrating that cerebrospinal fluid (CSF) LXA4 levels are reduced in dementia. Furthermore, LXA4 correlates with memory performance, Aβ, and brain-derived neurotrophic factor (BDNF) in the human CSF. Altogether, these results support a protective role for LXA4 in the brain that is dysregulated in aging and, to a greater extent, in dementia.
Methods
Study design and ethics
All mouse experiments were performed under protocols approved and supervised by the Institutional Animal Care and Use Committee of Federal University of Rio de Janeiro (CEUA-UFRJ) (protocol no. IBQM058). The sample size for each experiment was estimated by performing pilot studies and by previous experience with different experimental approaches. No algorithm or software was used to randomize animal subjects. Animal subjects were assigned to experimental groups by the researchers. Experiments with animal samples were performed in a blinded fashion. Experimental procedures involving human clinical data and CSF samples were approved by the Institutional Review Board (IRB) of Copa D’Or Hospital (protocol #47163715.0.0000.5249). Written informed consent was obtained from all volunteers. Samples were anonymized, and measurements were performed by trained investigators in a blinded fashion. All studies have been performed according to national and international ethical regulations and standards.
iPSC cultures
Three different lines of human-induced pluripotent stem cells were employed in this study—GM23279A iPSC line obtained commercially from Coriell Institute Biobank; CF2 line, generated from fibroblasts; and C15 line, obtained from urine cells. Cells were routinely tested for mycoplasma contamination and reprogrammed using the CytoTune 2.0 Sendai Reprogramming kit (Thermo Fisher Scientific, USA), and characterization of the reprogrammed cell lines was conducted by immunostaining of both iPSCs colonies and iPSCs-derived embryoid bodies for self-renewal and three germ layer markers as described [23, 24]. iPSCs were used to generate human neural progenitor cells (NPCs) by induction with retinoic acid for 18 days [25, 26]. When neural tube-like structures emerged, they were collected and replated on adherent dishes treated with 10 µg/mL Poly-L-ornithine and 2.5 µg/mL laminin (Thermo Fisher Scientific). After passages using Accutase, homogenous cultures were obtained [27]. NPCs were maintained and grown in DMEM/F12 supplemented with N2, 2% B27, 25 ng/mL bFGF, and 20 ng/mL EGF (Thermo Fisher Scientific), with medium changes every other day. The astrocyte cultures and mixed neuronal cultures were obtained from the differentiation of neural stem cells (NSCs), as described [28].
Primary cell cultures
Primary astrocyte, microglial and neuronal cultures were obtained from Swiss mice. Brain tissue was dissociated into single cells in DMEM-F12 (Invitrogen; Carlsbad, CA, USA) supplemented with glutamine (2 mM), penicillin and streptomycin (0.5 μg/mL, Hyclone, Logan UT, USA), amphotericin B (0.65 μM, Sigma–Aldrich, St. Louis, MO, USA) and 10% fetal bovine serum (FBS, Invitrogen; Invitrogen, Carlsbad, CA, USA). Cells were plated in 25 cm2 bottles pretreated with poly-L-lysine (Sigma–Aldrich, St. Louis, MO, USA). To generate astrocytes, cultures were maintained at 37 °C in an atmosphere of 5% CO2 for 7 days until confluence was achieved. Confluent cells were passaged to generate purified astrocyte cultures. Microglia were isolated on day 13 in vitro by shaking for 45–60 min and plated on adherent dishes supplemented with DMEM-F12 with 10% FBS and maintained for more 24 h. Hippocampal neurons were obtained from 16-days embryonic mice and maintained in Neurobasal medium (Invitrogen; Carlsbad, CA, USA) supplemented with B27 (Life Technologies, Carlsbad CA, USA), penicillin, streptomycin (0.5 μg/mL, Hyclone, Logan UT, USA), glutamine (2 mM), and fungizone (0.65 μM, Sigma–Aldrich, St. Louis, MO, USA). Cultures were maintained at 37 °C in a humidified atmosphere with 5% CO2 for 9 days in vitro. Cell homogenates were collected, centrifuged at 10,000 × g for 5 min at 4 °C, and used for LXA4 measurements.
Brain organoids
Brain organoids were generated from GM23279A induced pluripotent stem cell (iPSC) line as previously described [29]. Briefly, 9000 iPSC per well were plated on an ultralow attachment 96-well plate in hESC medium (20% knockout serum replacement; Life Technologies) containing 50 µM ROCKi (Y27632; Merck Millipore, USA) and 4 ng/ml b-FGF. The plate was spun, and cells were kept for 7 days to allow the formation of embryoid bodies (EBs). On the 7 following days, EBs were changed to ultralow attachment plates, incubated in a Matrigel bath for 1 h, and media changed from neuroinduction media (1% N2 supplement (Gibco), 1% GlutaMAX (Life Technologies), 1% MEM-NEAAs, 1% P/S, and 1 μg/ml heparin in DMEM/F12 (Life Technologies)) to differentiation media minus vitamin A (50% neurobasal medium, 0.5% N2, 1% B27 supplement without vitamin A, 1:100 2-mercapto-ethanol, 0.5% MEM-NEAA, 1% GlutaMAX, and 1:100 P/S in DMEM/F12). On day 15, organoids were transferred to agitation in six-well plates at 90 rpm, and media was changed to differentiation media plus vitamin A (50% neurobasal medium, 0.5% N2, 1% B27 supplement with vitamin A, 1:100 2-mercapto-ethanol, 0.5% MEM-NEAA, 1% GlutaMAX, and 1:100 P/S in DMEM/F12) and replaced every four days until ready for the experiment. For LXA4 measurements, 45–60 days in vitro brain organoids were used. For immunofluorescence experiments, brain organoids were fixed overnight on day 45 in 4% PFA, then dehydrated in 30% sucrose, frozen in optimal cutting temperature compound on dry ice, and sectioned (20 μm thickness) with a cryostat (Leica Biosystems, Germany).
Mouse strains
Swiss albino mice were provided by Fundação Oswaldo Cruz (FIOCRUZ); inbred C57BL/6 were provided by Charles River, and 5-LOX knockouts (and wild-type littermates) were provided by FIOCRUZ and kept in the animal facilities at the Federal University of Rio de Janeiro. Adult male mice were used throughout the study and tested during the light phase of the light cycle. Mice were maintained on a 12 h light/dark cycle with food and water ad libitum, with a maximum of 5 mice per cage.
In vivo treatments
Adult male Swiss mice received an intraperitoneal (i.p.) injection of 0.3 mg/kg lipopolysaccharide (LPS) or vehicle immediately after the contextual fear conditioning training session. One hour later, mice received an intracerebroventricular (i.c.v.) injection of either LXA4 (1 pmol) (Cayman Chemicals; #90410) or vehicle and were tested for memory 7 days later. For cytokine determination, WT or 5-LOX−/− mice received an intraperitoneal (i.p.) injection of 0.3 mg/kg lipopolysaccharide (LPS) or vehicle and were euthanized 4 h later. Brains were collected for ELISA.
Immunofluorescence
For immunofluorescence in brain organoids, frozen sections were rinsed with PBS and permeabilized with PBS-Triton 0.3% (PBST) for 15 min. Slides were then incubated with 0.01 M citrate buffer with 0.05% Tween 20 pH 6.0 for 10 min at 98 °C for antigen retrieval and blocked with blocking solution (PBS with 3% BSA) for 2 h at room temperature. Primary antibodies against 5-LOX (Cayman Chemical #160402, 1:100) or MAP2 (Sigma–Aldrich #M9942, 1:300) diluted in blocking solution were incubated at 4 °C overnight. Sections were then rinsed with PBS and incubated in AlexaFluor-conjugated IgG secondary antibody goat anti-mouse, rabbit, or goat (Invitrogen, 1:400) for 1 h at room temperature and washed three times for 5 min in PBS. For nuclear staining, sections were incubated with DAPI for 5 min. Slides were washed again three times for 5 min in PBS and then cover-slipped with Aqua-Poly/Mount (Polysciences Inc, Warrington, PA). Images were acquired using a Leica TCS SP8 confocal microscope. For immunofluorescence experiments in the mouse hippocampus, mice were transcardially perfused with saline and 4% formaldehyde. Brains were removed and post-fixed in 4% formaldehyde overnight, then submerged in 30% sucrose solution. Forty micrometer thick hippocampal cryosections were obtained, mounted on glass slides, and exposed to 0.01 M citrate buffer for antigen retrieval for 20 min at 60 °C. Tissue was permeabilized with 0.5% Triton X-100 in 50 mM ammonium chloride for 20 min at room temperature. Sections were then incubated in blocking solution (5% bovine serum albumin in PBST) for 60 min at room temperature and then incubated with anti-CB1R (Merck Millipore #209550, 1:1000) in blocking solution overnight at 4 °C. Sections were then rinsed with PBST and incubated with AlexaFluor-conjugated anti-rabbit IgG secondary antibodies (1:400) for 2 h at room temperature, followed by a short 5 min incubation in a DAPI solution (Molecular Probes). A 5 min incubation in 1% Sudan Black B prepared in 70% ethanol was applied to quench autofluorescence. Tissue was mounted on coverslips with Aqua-Poly/Mount (Polysciences Inc., Warrington, PA) and imaged on a Zeiss AxioImager M2 microscope. Image fluorescence was quantified on ImageJ [30] after the selection of the stratum pyramidale and stratum radiatum of hippocampal CA1 and CA3 subfields as regions of interest.
Human samples
CSF samples for this study were obtained from a cohort recruited at the D’Or Institute of Research and Education (IDOR) in Rio de Janeiro, Brazil. This cohort comprised healthy controls (HC; N = 25), and cognitively impaired subjects with diagnosis of either amnestic mild cognitive impairment (aMCI; N = 13), Alzheimer’s disease (N = 14), or dementia with Lewy bodies (DLB; N = 9). Inclusion criteria for this study were: age > 60 years; absence of other neurological conditions, neurodevelopmental or genetic diseases; native Brazilian Portuguese speakers; formal education ≥ 8 years; no restriction for MRI studies; no severe metabolic disease). All patients were evaluated with the same extensive clinical, neuropsychological, and neuroimaging investigation as described [31,32,33,34,35]. For demographics and biomarker information, see Table 1. The frequency of use of antidepressants or anti-inflammatory medication was not significantly different among groups. CSF samples were collected by lumbar puncture performed around 11 a.m. in all cases, to minimize circadian fluctuations. CSF was centrifuged, and the supernatant was collected, aliquoted, and immediately frozen at −80 °C. Before assays, samples were thawed and kept on ice until use. Samples and calibrators were run in duplicates.
ELISA
ELISA kits for LXA4 (#EA46) were from Oxford Biomedical Research (Rochester Hills, MI). For LXA4, mouse brains were harvested, and lipid extraction was performed with ethanol (5 μL/mg of wet tissue) followed by centrifugation for 5 min at 10,000 × g. The supernatant was applied directly into a double-sandwich ELISA kit, read at 650 nm, and normalized by wet tissue weight (g). Plasma and CSF samples and culture supernatants were applied to the ELISA kits according to the manufacturer’s instructions. ELISAs for IL-1β and IL-6 were performed with kits from R&D Systems (Minneapolis, MI). For cytokine measurements, brains were homogenized in RIPA buffer (Thermo Fisher, Waltham, MA) with protease and phosphatase inhibitors (Thermo Fisher, Waltham, MA), and protein concentration was determined by the bicinchoninic acid (BCA) assay. ELISA kits for Aβ42, and total tau (t-tau) were provided by Euroimmun (Lübeck, Germany), and experiments were run following the manufacturer’s instructions.
Inhibitory avoidance
The step-down inhibitory avoidance apparatus consisted of a box measuring 26 × 10 × 35 cm with a 10 × 10 × 4 cm platform placed in the center, surrounded by a floor made of parallel bronze bars and connected to a power source. For the training sessions, mice were placed on the platform, and when they stepped down with four paws onto the grid, they received a 0.7 mA foot shock for 2 s, and immediately returned to their home cage. For the test session (30 min or 24 h after training), animals were again placed on top of the platform, and latency to step down was recorded. For the protocol with multiple trials, a milder shock of 0.5 mA was used, and the procedure was repeated until the animal learned to remain on the platform for 180 s (criteria). For memory extinction, mice were conditioned in the step-down inhibitory avoidance task, as described above, only with a 1 mA foot shock intensity. Starting on the 12th day after conditioning, mice were submitted to successive extinction trials in 24 h intervals, where they were placed on the platform and allowed to step down onto the grid in the absence of a foot shock. The latency to step down was recorded, and mice were allowed to freely explore the apparatus for 30 s after every trial. Reduction in step-down latency across successive trials indicated extinction learning.
Contextual fear conditioning
To assess contextual fear memory consolidation, a two-phase protocol was used. Swiss mice were initially trained in the conditioning cage (40 × 25 × 30 cm) and were allowed to freely explore for 3 min followed by the application of a single foot shock (1 mA) for 2 s. Mice were kept for another 1 min in the cage and removed. Right after training, mice received an intraperitoneal injection of 0.3 mg/kg lipopolysaccharide (LPS) obtained from E. coli 0127:B8 (Sigma–Aldrich) or vehicle. One hour later, mice received an intracerebroventricular (i.c.v.) injection of either LXA4 (1 pmol) or vehicle. Seven days after training, mice were presented to the same cage for 5 min without receiving a foot shock. Freezing behavior was recorded automatically using the Freezing software (Panlab; Cornella, Spain). In all behavioral experiments, the experimenter was blinded to the groups tested.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 software (La Jolla, CA) or the IBM SPSS Statistics v. 26 (Armonk, NY). Differences between two independent groups were analyzed using Student’s t-test. When three or more independent experimental groups were compared, one- or two-way ANOVA was used, followed by appropriate post hoc tests, as stated in “Figure Legends”. For correlations, data distribution was initially assessed using Shapiro–Wilk Test. Given that significant deviation from normal distribution was observed, after adjustment for age, partial rank correlations were performed to assess the relationship between CSF LXA4 levels and clinical/biomarker variables. The significance level was set at 0.05. The predictive power of CSF LXA4, alone or in combination with Aβ42, to identify clinical AD was tested by plotting receiver operating characteristic (ROC) curves, widely used to determine the diagnostic potential of biomarkers, and determining the area under the curve (AUC) with a confidence interval of 0.95.
Results
LXA4 is produced by cultured neurons and microglia, and by human brain organoids
Lipoxins, including LXA4, are locally produced by immune cells at sites of inflammation or systemically [13]. Although we and others have previously reported that LXA4 is bioactive in the brain [16, 17, 21], whether brain cells comprise a source for LXA4 remains unknown. We first used primary cultures to investigate whether brain cells produced LXA4 at detectable levels. We found that mouse hippocampal neurons and microglia had a significantly higher content of LXA4 than astrocytes (Fig. 1a). We next used human-induced pluripotent stem cells (iPSCs) to derive human neural progenitor cell (NPC), neuron, astrocyte or microglial-like cultures to determine the levels of LXA4. We found that human iPSC-derived neurons produce considerably higher levels of LXA4 than astrocytes or NPCs (Fig. 1a). Furthermore, in line with mouse results, levels of human microglia-sourced LXA4 are comparable to human neurons, suggesting microglia as key producers of LXA4 (Fig. 1a). To further ascertain whether neuronal LXA4 would be present in more complex systems, we performed ELISA measurements of LXA4 in the cellular content and conditioned media from 45- to 60-day-old human brain organoids derived from iPSC. At that stage, human brain organoids express a biochemical profile, patterning, and structural organization similar to what is observed in the human embryonic brain [29]. We found that LXA4 is present in both homogenates and culture media from human brain organoids (Fig. 1b). The relatively higher content of LXA4 in the homogenate may suggest that LXA4 release and extracellular content are tightly regulated. Immunofluorescence experiments to label 5-LOX further revealed prominent 5-LOX labeling in microtubule-associated protein 2 (MAP2)-positive cells (Fig. 1c), confirming that human neurons typically located in the outer layers of human brain organoids express 5-LOX. Together, our results raise the possibility that neurons and microglia are important sources of LXA4 in the human brain.
a Levels of lipoxin A4 (LXA4) in primary cultures enriched in astrocytes, microglia or neurons derived from mice (left) (N = 5 for astrocytes and neurons, 6 for microglia) or in human iPSC-derived astrocytes, microglia, neurons, or neural progenitor cells (NPCs) (N = 3 for astrocytes and neurons, 6 for microglia, and 10 for NPCs). Unpaired two-tailed one-way ANOVA with Šidák post hoc test (***p < 0.001; ****p < 0.0001; n.s. nonsignificant). n.d. not detected. b Levels of LXA4 in the lysates (green bar) or conditioned media (brown bar) of human brain organoids (N = 8 for lysates, 4 for conditioned media). c Representative images of immunofluorescence experiments (5-lipoxygenase, 5-LOX immunoreactivity: green; microtubule-associated protein 2, MAP2 immunoreactivity: red; DAPI: blue; N = 3) in human iPSC-derived brain organoids. Scale bar: 10 μm.
Aging reduces systemic and brain LXA4 levels in mice
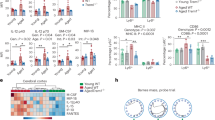
Since aging impairs systemic response to injuries [36, 37] and renders the brain vulnerable to neurodegenerative conditions [38], we next determined whether levels of LXA4 would be modified by aging in mice. We found that plasma and brain LXA4 levels were reduced in 12-month-old male Swiss mice compared to young 3-month-old controls (Fig. 2a, b). This is accompanied by evident short-term memory impairment in the inhibitory avoidance memory test, as aged mice presented reduced latency to step down from the platform in the test sessions (Fig. 2c). Results indicate that reduced brain and peripheral LXA4 levels concur with cognitive deficits in aged mice.
a, b Levels of lipoxin A4 (LXA4) in plasma (a) and brain (b) of young (3 month old) or aged (12 month old) Swiss mice. (Plasma: N = 12 for 3 mo, N = 15 for 12 mo; Brain: N = 7 for 3 mo, N = 15 for 12 mo mice). c Step-down latency of young or aged mice in the inhibitory avoidance task to assess short-term memory (STM: 1 h after training; N = 20 for 3 mo; N = 15 for 12 mo mice). d Number of trials required for each mouse to reach the criteria during the training session in the inhibitory avoidance fear task (N = 23 for WT; N = 13 for 5-LOX−/−). e, f Step-down latency of 5-LOX−/− or WT mice (3 month old) in the inhibitory avoidance task to assess short-term (e; STM: 1 h after training; N = 7 WT and N = 15 5-LOX−/− mice) or long-term memory (f; LTM: 24 h after training; N = 23 for WT; N = 13 for 5-LOX−/−). For a, b, and d, two-tailed unpaired Student’s t-test. For c, e, and f, two-tailed unpaired Mann–Whitney (*p < 0.05; ****p < 0.0001). Graphs show means ± standard error of the mean (SEM). Each dot represents an individual. g Fear memory extinction assessed by step-down latency of 5-LOX−/− or WT after repeated test sessions 12–15 days after original conditioning session (N = 10 WT and N = 10 5-LOX−/− mice). Repeated measures one-way ANOVA with Šidák post hoc test (*p < 0.05). Graphs show means ± SEM.
Suppression of 5-LOX mimics age-associated memory loss in mice
We next investigated whether reductions in 5-LOX would be causally implicated in memory impairments in mice. Therefore, we tested memory performance in 3-month-old (young) 5-LOX homozygous knockout mice (5-LOX−/−), which show reduced circulating LXA4 levels [21], and their respective wild-type littermates (WT) in the inhibitory avoidance memory task. We used adult 5-LOX−/−mice in these experiments to specifically dissect the roles of 5-LOX while avoiding potential confounders triggered by normal aging. Control experiments revealed no differences in body weight (Supplementary Fig. S1a) or food and water intake (Supplementary Fig. 1b, c) across genotypes. Additionally, levels of hippocampal CB1 receptors and LPS-induced interleukin 1β (IL-1β) production, as a proxy of brain inflammatory response, were similar in 5-LOX−/− and WT mice (Supplementary Fig. 1d–f).
5-LOX−/− mice took significantly more learning sessions in the inhibitory avoidance task to reach the criteria than WT (Fig. 2d). While WT showed normal memory performance, 5-LOX−/− mice presented impaired short-term, but not long-term memory (Fig. 2e, f). Furthermore, 5-LOX−/− mice had impaired extinction of learned fear memory (Fig. 2g), which is in further agreement with learning deficits. Altogether, these results demonstrate that 5-LOX supports proper cognitive function and indicate that reductions in LXA4 may render the brain vulnerable to dysfunction and memory impairment.
Administration of LXA4 attenuates inflammatory responses and memory loss
Our results so far raised the notion that LXA4-mediated signaling could be part of a protective mechanism against age-related cognitive decline, which is, at least in part, mediated by inflammation [39]. We then sought to determine whether the administration of LXA4 would protect against impairments in memory consolidation induced by an inflammatory injury. Mice were trained in a contextual fear conditioning paradigm and received a post-training intraperitoneal (i.p.) injection of 0.3 mg/kg lipopolysaccharide (LPS) or vehicle. One hour after LPS, mice received an intracerebroventricular (i.c.v.) injection of either LXA4 (1 pmol) or vehicle and were tested for memory 7 days later. We found that i.c.v. LXA4 administration rescued impaired contextual fear memory consolidation in LPS-injected mice (Fig. 3a), advocating for a neuroprotective effect via reduction of inflammatory pathways.
a Freezing (seconds) of control or LPS-injected mice (0.3 mg/kg) treated with vehicle or 1 pmol LXA4 (i.c.v) in the contextual fear conditioning task. b–e Brain and plasma levels of IL-1β (b, c) or IL-6 (d, e) in control or LPS-injected mice (0.3 mg/kg; i.p.) treated with vehicle or 1 pmol LXA4 (i.c.v). Two-tailed unpaired two-way ANOVA followed by Holm–Šidák post hoc test (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001). Graphs show means ± standard error of the mean (SEM).
We thus attempted to determine the impact of LXA4 on LPS-induced inflammatory cytokine production. I.c.v. LXA4 administration reduced levels of IL-1β (Fig. 3b, c) and interleukin 6 (IL-6) (Fig. 3d, e) in the brains and plasma of LPS-treated mice. These results indicate that LXA4 attenuates peripheral and brain inflammation, and rescues inflammation-induced cognitive defects in mice.
Cerebrospinal fluid LXA4 declines with aging and dementia in humans
To test the potential relevance of LXA4 in humans, we investigated LXA4 levels in the cerebrospinal fluid (CSF) of a cohort of human subjects in a memory clinic. They were diagnosed as mild cognitive impairment (MCI), AD, dementia with Lewy bodies (DLB), or controls (see Table 1 for demographics and biomarker information). We initially found that LXA4 declines with aging in human CSF in this cohort (Fig. 4a), in line with our mouse studies. We further determined that LXA4 levels were markedly decreased in subjects with AD or DLB, but not in MCI, as compared to controls (Fig. 4b). Together, these results support the notion that aging leads to a reduction in brain LXA4 levels. The decrease in LXA4-mediated protective mechanisms may lead to vulnerability to cognitive diseases and, presumably, neurodegeneration.
a Correlation between age (in years) and CSF LXA4 in human subjects. b CSF levels of LXA4 in AD and DLB patients compared to healthy controls or amnestic MCI patients (N = 25 controls, 13 aMCI, 14 AD, 9 LBD patients). c CSF LXA4 categorized by clinical dementia rating (CDR) (N = 7 CDR 0; N = 40 CDR 0.5; N = 13 CDR 1). Two-tailed unpaired one-way ANOVA followed by Holm–Šidák post hoc test (**p < 0.01; ns nonsignificant). Graphs show means ± standard error of the mean (SEM). d–f Correlations between LXA4 and MMSE scores (d), CSF BDNF (e), or Aβ42 (f) levels in human subjects. Lines represent partial rank correlations (r and p-values as indicated in graphs), adjusted for age, and the confidence interval is represented as gray shade. g Receiver operating characteristic curves for diagnostic based on Aβ42 alone (red line) or LXA4*Aβ42 (blue line); confidence interval: 0.95; p < 0.001. HC healthy controls, white symbols, aMCI amnestic mild cognitive impairment, gray symbols, AD Alzheimer’s disease, black symbols, DLB dementia with Lewy bodies, golden symbols, AUC area under the curve.
Cerebrospinal fluid LXA4 correlates with memory performance, BDNF, and Aβ42 levels in humans
We then investigated whether CSF LXA4 would associate with cognitive performance and the levels of markers relevant to AD. We initially found that individuals with worse clinical dementia ratings (CDR) had lower levels of LXA4 in the CSF (Fig. 4c). CSF LXA4 showed positive correlations with mini-mental state exam (MMSE) scores, a proxy for global cognition in humans (Fig. 4d). We further found that LXA4 positively correlates with BDNF, a neurotrophin essential for memory function [40, 41], in the CSF (Fig. 4e). These results indicate that central LXA4 actions may favor brain homeostasis and cognition.
We next addressed potential associations between CSF LXA4 and AD biomarkers (Aβ42 and tau). We found that CSF LXA4 positively associates with CSF Aβ42 (Fig. 4f), but not with tau (Supplementary Fig. 2). Finally, the combination of CSF LXA4 and Aβ42 slightly increased the AUC for AD diagnostics in a receiver operating characteristic (ROC) curve (Fig. 4g). These results suggest that declines in CSF LXA4 correlate with brain Aβ42 accumulation, possibly facilitating its neurotoxic actions.
Discussion
Age-related cognitive impairment and dementia are among the causes of significant disability in the elderly, and effective interventions are still not available. Nonetheless, it is well established that loss of immune homeostasis and aberrant inflammatory responses comprise significant factors predisposing individuals to brain dysfunction, cognitive impairment, and dementia [42,43,44,45]. Here we provide evidence suggesting that LXA4 is part of an endogenous protective mechanism that decreases with aging and renders the brain vulnerable to memory failure and dementia.
We first addressed a long-standing question of whether brain cells produce LXA4. Previous evidence indicated that rodents and human neurons and microglia express 5-LOX, the enzyme required for LXA4 synthesis [18, 19, 46]. Nonetheless, direct evidence for LXA4 production in brain cells was lacking. We found that mouse and human neurons and microglia in culture actively produce and release LXA4, whereas astrocytes produce smaller amounts of LXA4. We also found that human brain organoids, a more complex experimental model, produce and release LXA4. These results suggest that neurons and microglia could act as local sources of LXA4 in the brain and builds upon our previous data showing that LXA4 is present in the brain despite the absence of its canonical ALX receptor, at least under physiological conditions [21].
We and others have provided initial evidence suggesting that peripheral LXA4 levels are reduced with aging in mice and humans. While we have previously shown that 12-month-old mice present reduced plasma levels of LXA4 as compared to 3-month-old mice [16], Gangemi et al. demonstrated that urinary LXA4 is markedly reduced in aged humans [36]. We now confirmed these previous results in plasma and extended our studies to demonstrate that central LXA4 also declines with age and accompanies memory impairment in mice.
We determined that 5-LOX−/− mice, which present reduced plasma and brain LXA4 content, present learning impairment in an inhibitory avoidance task. We decided to use 3-month-old 5-LOX−/− mice (instead of aged subjects) in these experiments to circumvent a potential source of confusion with other aspects related to the aging process. For instance, 5-LOX−/− mice have been reported to develop additional age-dependent behavioral alterations, such as anxiety-like behavior [47], which are not present in young mice [16]. Future studies are warranted to determine the relative contributions of circulating and brain LXA4 to cognition by ablating 5-LOX in specific sources of LXA4 (i.e., neurons, microglia, neutrophil, and platelets) and also to thoroughly investigate the contributions of other 5-LOX-dependent lipid mediators in cognition, especially considering that LXA4 is not the only metabolite whose synthesis depends on 5-LOX.
We acknowledge that 5-LOX was previously shown to have a deleterious impact in mouse models of AD and tauopathy [48,49,50,51]. These apparently controversial findings could be explained by altered inflammatory profiles developed by these transgenic models [52,53,54,55], likely resulting in dyshomeostasis of other lipid derivatives controlled by 5-LOX, including leukotrienes [56].
Stimulation of LXA4 signaling through aspirin-triggered lipoxin (ATL) was shown to promote beneficial actions, including reduced microglial reactivity, less Aβ42 accumulation and tau phosphorylation, and attenuation of memory impairment in mouse models of AD [17, 22]. Notably, LXA4 was reported to attenuate Aβ-induced memory impairment through CB1 receptors [21]. Our findings further demonstrate that brain administration of LXA4 prevents inflammation-induced cytokine upregulation and memory consolidation defects in mice. Results suggest that preserving LXA4 signaling across adulthood might be beneficial to ward off persistent inflammation, cognitive decline, and AD risk at later stages of life.
We have previously shown that LXA4 acts as an allosteric agonist of CB1 receptors in the brain, enhancing the affinity of CB1 receptors to anandamide [21]. Given that endocannabinoid signaling through CB1 receptors is essential for proper brain functions [57,58,59,60], locally produced LXA4 could serve as a means of intercellular communication in the CNS to fuel synaptic plasticity, potentiate astrocyte metabolism and microglial surveillance. Conversely, reduced brain LXA4 could explain, at least in part, compromised CB1 receptor signaling, which may then translate into memory impairment [61].
Our results show that CSF LXA4 is considerably reduced in patients with dementia (AD and DLB), conferring translational relevance to rodent studies. Reductions in CSF LXA4 were consistent despite the pathological heterogeneity indicated by varying levels of CSF Aβ42 and tau. Significant correlations of CSF LXA4 with BDNF, cognitive function, and CDR in humans also indicate potential pro-mnemonic actions of central LXA4 and highlight the need for further translational investigation of the underlying mechanisms. An intriguing observation relies on the positive correlation of CSF LXA4 with Aβ42, but not with tau, in humans. This suggests that brain Aβ accumulation (as assessed by lower CSF Aβ42), an early and predictive marker of AD [62,63,64], may associate with reduced LXA4 in the CNS independently of tau pathology (as determined by CSF tau). An exciting perspective will be to clarify the interrelation of Aβ42, tau, and LXA4 in the context of the AT(N) clinical research framework [65].
A combination of CSF LXA4 and Aβ42 resulted in a modestly increased AUC for AD diagnostic, although the specificity for Aβ42 alone was higher at higher sensitivities. From our current investigation, the use of Aβ42 alone would result in more true positive rates. This could be seen as a potential limitation, in addition to the reduced sample size in the clinical cohort. Nonetheless, these results encourage additional investigation to determine whether the incorporation of LXA4 measurements into a broader CSF biomarker panel can aid in the discrimination of a subset of AD cases, thereby improving overall diagnostics.
Biological sex is a key variable in the epidemiology and clinical phenotypes of AD, with women being more affected than men [66], and intrinsic sex differences in inflammation resolution [67] and aspirin-induced LXA4 [68] have been reported. Therefore, it will be important to replicate our mouse studies, which only used male subjects, in females in the future. Furthermore, sex was considered a covariable in the correlations obtained from our human study. Although our data indicate similar trends of reduction in CSF LXA4 for both men and women (data not shown), future studies in larger cohorts with proper statistical power are warranted to ascertain potential sex differences related to CSF LXA4 in healthy subjects and in dementia patients.
Activation of the innate immune system appears to mediate cognitive decline and AD pathogenesis [39, 69, 70]. Indeed, aberrant cytokine release [71, 72] and abnormal innate immune response to brain Aβ deposition have been reported in mouse models and patients of AD [73, 74]. Infiltration of immune cells, such as neutrophils [75], into the brain may shift cellular responses towards reduced LXA4 production and, consequently, increased neurotoxicity in AD. It is noteworthy that LXA4 is a potent antagonist of leukotriene receptors [76], and recent evidence supports that the use of leukotriene receptor antagonists brings cognitive benefits to AD-related dementia [77]. This suggests an indirect effect through which reduced central LXA4 contributes to worse cognition in AD.
We hypothesize that LXA4 deploys a dual mechanism as an anti-inflammatory and neuroprotective agent, thereby contributing to the resilience of the central nervous system against disease-associated insults, such as Aβ42, in AD. Hence, LXA4 levels may be useful as a biomarker of brain vulnerability, and its decreased levels may indicate a homeostatic breakdown. Since a significant part of the CB1 receptor-related effects of anandamide is due to synergistic LXA4 interaction [21], the vulnerability window caused by age-associated LXA4 reduction might be conceived as part of the “endocannabinoid deficiency syndrome” hypothesis [78]. This concept advocates that under circumstances of relatively low endocannabinoid signaling, certain inflammation-linked injuries, such as fibromyalgia, inflammatory bowel syndrome, and migraine, become more incident. Here we propose to extend this concept to include neurodegeneration and dementia.
In summary, our findings support the notion that LXA4 signaling may comprise an endogenous protective mechanism that renders the brain vulnerable to exacerbated inflammation and neurodegenerative factors (i.e., Aβ42 deposition). Preventing declines in LXA4 levels may preserve endocannabinoid signaling, brain homeostasis, and cognition, thereby contributing to reduced odds of dementia. Future studies are needed to establish the intricate signaling pathways initiated by brain actions of LXA4, as well as to test whether stimulation of LXA4 signaling (or the endocannabinoid system, where LXA4 acts as a co-agonist [21]) is effective in delaying cognitive decline in aged humans.
References
Selles MC, Oliveira MM, Ferreira ST. Brain inflammation connects cognitive and non-cognitive symptoms in Alzheimer’s disease. J Alzheimers Dis. 2018;64:S313–S327.
De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 2014;63:1–11.
Lourenco MV, Ferreira ST, De Felice FG. Neuronal stress signaling and eIF2alpha phosphorylation as molecular links between Alzheimer’s disease and diabetes. Prog Neurobiol. 2015;129:37–57.
Clarke JR, Lyra ESNM, Figueiredo CP, Frozza RL, Ledo JH, Beckman D, et al. Alzheimer-associated Abeta oligomers impact the central nervous system to induce peripheral metabolic deregulation. EMBO Mol Med. 2015;7:190–210.
Lourenco MV, Clarke JR, Frozza RL, Bomfim TR, Forny-Germano L, Batista AF, et al. TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s β-amyloid oligomers in mice and monkeys. Cell Metab. 2013;18:831–43.
Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405.
Yin F, Sancheti H, Patil I, Cadenas E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic Biol Med. 2016;100:108–22.
Lopez-Rodriguez AB, Hennessy E, Murray CL, Nazmi A, Delaney HJ, Healy D, et al. Acute systemic inflammation exacerbates neuroinflammation in Alzheimer’s disease: IL-1beta drives amplified responses in primed astrocytes and neuronal network dysfunction. Alzheimers Dement. 2021;17:1735–55.
Lyra-e-Silva NM, Goncalves RA, Pascoal TA, Lima-Filho RAS, Resende EPF, Vieira ELM, et al. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl Psychiatry. 2021;11:251.
De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer’s disease? Alzheimers Dement. 2014;10:S26–S32.
Lourenco MV, Ledo JH. Targeting Alzheimer’s pathology through PPARγ signaling: modulation of microglial function. J Neurosci. 2013;33:5083–4.
Chandrasekharan JA, Sharma-Walia N. Lipoxins: nature’s way to resolve inflammation. J Inflamm Res. 2015;8:181–92.
Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation Immunity. 2014;40:315–27.
Martini AC, Forner S, Bento AF, Rae GA. Neuroprotective effects of lipoxin A4 in central nervous system pathologies. Biomed Res Int. 2014;2014:316204.
Chiang N, Takano T, Arita M, Watanabe S, Serhan CN. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br J Pharmacol. 2003;139:89–98.
Leo LM, Almeida-Correa S, Canetti CA, Amaral OB, Bozza FA, Pamplona FA. Age-dependent relevance of endogenous 5-lipoxygenase derivatives in anxiety-like behavior in mice. PLoS ONE. 2014;9:e85009.
Medeiros R, Kitazawa M, Passos GF, Baglietto-Vargas D, Cheng D, Cribbs DH, et al. Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces Alzheimer disease-like pathology in mice. Am J Pathol. 2013;182:1780–9.
Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernandez-Lopez D, Pradillo JM, et al. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–84.
Jatana M, Giri S, Ansari MA, Elango C, Singh AK, Singh I, et al. Inhibition of NF-kappaB activation by 5-lipoxygenase inhibitors protects brain against injury in a rat model of focal cerebral ischemia. J Neuroinflammation. 2006;3:12.
Derada Troletti C, Enzmann G, Chiurchiu V, Kamermans A, Tietz SM, Norris PC, et al. Pro-resolving lipid mediator lipoxin A4 attenuates neuro-inflammation by modulating T cell responses and modifies the spinal cord lipidome. Cell Rep. 2021;35:109201.
Pamplona FA, Ferreira J, Menezes de Lima O Jr., Duarte FS, Bento AF, Forner S, et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci USA. 2012;109:21134–9.
Dunn HC, Ager RR, Baglietto-Vargas D, Cheng D, Kitazawa M, Cribbs DH, et al. Restoration of lipoxin A4 signaling reduces Alzheimer’s disease-like pathology in the 3xTg-AD mouse model. J Alzheimers Dis. 2015;43:893–903.
Sochacki J, Devalle S, Reis M, de Moraes Maciel R, da Silveira Paulsen B, Brentani H, et al. Generation of iPS cell lines from schizophrenia patients using a non-integrative method. Stem Cell Res. 2016;17:97–101.
Sochacki J, Devalle S, Reis M, Fontenelle LF, Rehen S. Generation of urine iPS cell line from a patient with obsessive-compulsive disorder using a non-integrative method. Stem Cell Res. 2016;17:107–10.
Paulsen BS, de Moraes Maciel R, Galina A, Souza da Silveira M, dos Santos Souza C, Drummond H, et al. Altered oxygen metabolism associated to neurogenesis of induced pluripotent stem cells derived from a schizophrenic patient. Cell Transpl. 2012;21:1547–59.
Baharvand H, Mehrjardi N-Z, Hatami M, Kiani S, Rao M, Haghighi M-M. Neural differentiation from human embryonic stem cells in a defined adherent culture condition. Int J Dev Biol. 2007;51:371–8.
Dakic V, Maciel RM, Drummond H, Nascimento JM, Trindade P, Rehen SK. Harmine stimulates proliferation of human neural progenitors. PeerJ. 2016;4:e2727.
Yan Y, Shin S, Jha BS, Liu Q, Sheng J, Li F, et al. Efficient and rapid derivation of primitive neural stem cells and generation of brain subtype neurons from human pluripotent stem cells. Stem Cells Transl Med. 2013;2:862–70.
Goto-Silva L, Ayad NME, Herzog IL, Silva NP, Lamien B, Orlande HRB, et al. Computational fluid dynamic analysis of physical forces playing a role in brain organoid cultures in two different multiplex platforms. BMC Dev Biol. 2019;19:3.
Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;2004:1–7.
Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. 2019;25:165–75.
Drummond C, Coutinho G, Monteiro MC, Assuncao N, Teldeschi A, Souza AS, et al. Narrative impairment, white matter damage and CSF biomarkers in the Alzheimer’s disease spectrum. Aging 2019;11:9188–208.
Lourenco MV, Ribeiro FC, Sudo FK, Drummond C, Assuncao N, Vanderborght B, et al. Cerebrospinal fluid irisin correlates with amyloid-beta, BDNF, and cognition in Alzheimer’s disease. Alzheimer’s Dement: Diagnosis, Assess Dis Monit. 2020;12:e12034.
Drummond C, Coutinho G, Fonseca RP, Assuncao N, Teldeschi A, de Oliveira-Souza R, et al. Deficits in narrative discourse elicited by visual stimuli are already present in patients with mild cognitive impairment. Front Aging Neurosci. 2015;7:96.
Lourenco MV, Ribeiro FC, Santos LE, Beckman D, Melo HM, Sudo FK, et al. Cerebrospinal fluid neurotransmitters, cytokines, and chemokines in Alzheimer’s and Lewy body diseases. J Alzheimer’s Dis. 2021;82:1067–74.
Gangemi S, Pescara L, D’Urbano E, Basile G, Nicita-Mauro V, Davi G, et al. Aging is characterized by a profound reduction in anti-inflammatory lipoxin A4 levels. Exp Gerontol. 2005;40:612–4.
Yousef H, Czupalla CJ, Lee D, Chen MB, Burke AN, Zera KA, et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat Med. 2019;25:988–1000.
Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature 2004;430:631–9.
Herrup K. Reimagining Alzheimer’s disease-an age-based hypothesis. J Neurosci. 2010;30:16755–62.
Buchman AS, Yu L, Boyle PA, Schneider JA, De Jager PL, Bennett DA. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology 2016;86:735–41.
Forlenza OV, Diniz BS, Teixeira AL, Radanovic M, Talib LL, Rocha NP, et al. Lower cerebrospinal fluid concentration of brain-derived neurotrophic factor predicts progression from mild cognitive impairment to Alzheimer’s disease. Neuromolecular Med. 2015;17:326–32.
Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–72.
Czirr E, Wyss-Coray T. The immunology of neurodegeneration. J Clin Invest. 2012;122:1156–63.
Anita NZ, Zebarth J, Chan B, Wu CY, Syed T, Shahrul D, et al. Inflammatory markers in type 2 diabetes with vs. without cognitive impairment; a systematic review and meta-analysis. Brain Behav Immun. 2022;100:55–69.
Frozza RL, Lourenco MV, De Felice FG. Challenges for Alzheimer’s disease therapy: insights from novel mechanisms beyond memory defects. Front Neurosci. 2018;12:37.
Michael J, Unger MS, Poupardin R, Schernthaner P, Mrowetz H, Attems J, et al. Microglia depletion diminishes key elements of the leukotriene pathway in the brain of Alzheimer’s Disease mice. Acta Neuropathol Commun. 2020;8:129.
Joshi YB, Pratico D. Knockout of 5-lipoxygenase results in age-dependent anxiety-like behavior in female mice. PLoS ONE. 2011;6:e29448.
Giannopoulos PF, Chu J, Joshi YB, Sperow M, Li JG, Kirby LG, et al. Gene knockout of 5-lipoxygenase rescues synaptic dysfunction and improves memory in the triple-transgenic model of Alzheimer’s disease. Mol Psychiatry. 2014;19:511–8.
Chu J, Pratico D. Pharmacologic blockade of 5-lipoxygenase improves the amyloidotic phenotype of an Alzheimer’s disease transgenic mouse model involvement of gamma-secretase. Am J Pathol. 2011;178:1762–9.
Vagnozzi AN, Giannopoulos PF, Pratico D. The direct role of 5-lipoxygenase on tau pathology, synaptic integrity and cognition in a mouse model of tauopathy. Transl Psychiatry. 2017;7:1288.
Valera E, Dargusch R, Maher PA, Schubert D. Modulation of 5-lipoxygenase in proteotoxicity and Alzheimer’s disease. J Neurosci. 2013;33:10512–25.
Marchese M, Cowan D, Head E, Ma D, Karimi K, Ashthorpe V, et al. Autoimmune manifestations in the 3xTg-AD model of Alzheimer’s disease. J Alzheimers Dis. 2014;39:191–210.
Munch G, Apelt J, Rosemarie Kientsch E, Stahl P, Luth HJ, Schliebs R. Advanced glycation endproducts and pro-inflammatory cytokines in transgenic Tg2576 mice with amyloid plaque pathology. J Neurochem. 2003;86:283–9.
Bellucci A, Westwood AJ, Ingram E, Casamenti F, Goedert M, Spillantini MG. Induction of inflammatory mediators and microglial activation in mice transgenic for mutant human P301S tau protein. Am J Pathol. 2004;165:1643–52.
Lopez-Gonzalez I, Aso E, Carmona M, Armand-Ugon M, Blanco R, Naudi A, et al. Neuroinflammatory gene regulation, mitochondrial function, oxidative stress, and brain lipid modifications with disease progression in Tau P301S transgenic mice as a model of frontotemporal lobar degeneration-tau. J Neuropathol Exp Neurol. 2015;74:975–99.
Giannopoulos PF, Chiu J, Pratico D. Antileukotriene therapy by reducing tau phosphorylation improves synaptic integrity and cognition of P301S transgenic mice. Aging Cell. 2018;17:e12759.
Robin LM, Oliveira da Cruz JF, Langlais VC, Martin-Fernandez M, Metna-Laurent M, Busquets-Garcia A, et al. Astroglial CB1 receptors determine synaptic D-serine availability to enable recognition memory. Neuron 2018;98:935–44.e935.
Benard G, Massa F, Puente N, Lourenco J, Bellocchio L, Soria-Gomez E, et al. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat Neurosci 2012;15:558–64.
Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron 2012;76:70–81.
Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 2004;431:312–6.
Albayram O, Alferink J, Pitsch J, Piyanova A, Neitzert K, Poppensieker K, et al. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc Natl Acad Sci USA. 2011;108:11256–61.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92.
Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28.
Krance SH, Cogo-Moreira H, Rabin JS, Black SE, Swardfager W, Alzheimer’s Disease Neuroimaging I. Reciprocal predictive relationships between amyloid and tau biomarkers in Alzheimer’s disease progression: an empirical model. J Neurosci. 2019;39:7428–37.
Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione, et al. Sex differences in Alzheimer disease—the gateway to precision medicine. Nat Rev Neurol. 2018;14:457–69.
Rathod KS, Kapil V, Velmurugan S, Khambata RS, Siddique U, Khan S, et al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J Clin Invest. 2017;127:169–82.
Chiang N, Hurwitz S, Ridker PM, Serhan CN. Aspirin has a gender-dependent impact on antiinflammatory 15-epi-lipoxin A4 formation: a randomized human trial. Arterioscler Thromb Vasc Biol. 2006;26:e14–17.
Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer’s disease. Nat Immunol. 2015;16:229–36.
De Felice FG, Lourenco MV. Brain metabolic stress and neuroinflammation at the basis of cognitive impairment in Alzheimer’s disease. Front Aging Neurosci. 2015;7:94.
Bawa KK, Krance SH, Herrmann N, Cogo-Moreira H, Ouk M, Yu D, et al. A peripheral neutrophil-related inflammatory factor predicts a decline in executive function in mild Alzheimer’s disease. J Neuroinflammation. 2020;17:84.
Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psych. 2010;68:930–41.
Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21:880–6.
Cruz Hernandez JC, Bracko O, Kersbergen CJ, Muse V, Haft-Javaherian M, Berg M, et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci. 2019;22:413–20.
Baik SH, Cha MY, Hyun YM, Cho H, Hamza B, Kim DK, et al. Migration of neutrophils targeting amyloid plaques in Alzheimer’s disease mouse model. Neurobiol Aging. 2014;35:1286–92.
Gronert K, Martinsson-Niskanen T, Ravasi S, Chiang N, Serhan CN. Selectivity of recombinant human leukotriene D4, leukotriene B4, and lipoxin A4 receptors with aspirin-triggered 15-epi-LXA4 and regulation of vascular and inflammatory responses. Am J Pathol. 2001;158:3–9.
Xiong LY, Ouk M, Wu CY, Rabin JS, Lanctot KL, Herrmann N, et al. Leukotriene receptor antagonist use and cognitive decline in normal cognition, mild cognitive impairment, and Alzheimer’s dementia. Alzheimers Res Ther. 2021;13:147.
Russo EB. Clinical endocannabinoid deficiency reconsidered: current research supports the theory in migraine, fibromyalgia, irritable bowel, and other treatment-resistant syndromes. Cannabis Cannabinoid Res. 2016;1:154–65.
Acknowledgements
We thank the IDOR clinical and imaging staff for patients’ recruitment and diagnostic investigation.
Funding
This work was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) to FAP, HCCFN, and MVL, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to FAP and MVL, Alzheimer’s Association (AARG-D-615714) to MVL, Serrapilheira Institute (R-2012-37967) to MVL, and intramural grants from the D’Or Institute for Research and Education (IDOR) and Rede D’Or São Luiz Hospital Network.
Author information
Authors and Affiliations
Contributions
FAP and FTM designed the study. FAP, MVL, FCR, GV, FKS, ARI, CM, MGC, PFL, KK, IMO, LML, BP, GC, CD, NA, BV, and CC performed research. FAP, MVL, FKS, ARI, BP, and BV analyzed data. FAP, MVL, CC, HCCFN, SKR, STF, FAB, and FTM contributed reagents, materials, animals, and analysis tools. FAP, MVL, STF, FAB, and FTM analyzed and discussed results. FAP and MVL wrote the manuscript with input from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pamplona, F.A., Vitória, G., Sudo, F.K. et al. Age-linked suppression of lipoxin A4 associates with cognitive deficits in mice and humans. Transl Psychiatry 12, 439 (2022). https://doi.org/10.1038/s41398-022-02208-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-022-02208-1
This article is cited by
-
Dysregulation of neuroprotective lipoxin pathway in astrocytes in response to cytokines and ocular hypertension
Acta Neuropathologica Communications (2024)