Abstract
Adiponectin, an adipokine secreted by adipocytes, has anti-atherosclerotic and antithrombotic activities. AdipoRon is synthetic small molecule adiponectin receptor agonist. In this study, we investigated the effect of AdipoRon on platelet activation and thrombus formation. Washed human platelets were prepared from the peripheral blood of healthy donors. In a series of in vitro platelet functional assays, pre-treatment with AdipoRon (10, 20, 40 µg/mL) dose-dependently inhibited the aggregation, granule secretion and spreading of washed human platelets. We showed that AdipoRon (20, 40 µg/mL) significantly inhibited AMPK, Syk, PLCγ2, PI3K, Akt, p38-MAPK and ERK1/2 signalling pathways in washed human platelets. In addition, we demonstrated that the phosphorylation of CKII at Tyr255 was an important mechanism of the integrin αIIbβ3-mediated platelet activation. Meanwhile, AdipoR1 deficiency impaired the inhibitory effect of AdipoRon on mouse platelets. In ferric chloride-induced carotid injury model, injection of AdipoRon (5 or 12.5 mg/kg, iv) significantly attenuated arterial thrombosis. In conclusion, AdipoRon attenuates platelet function via the AdipoR1/AMPK/CKII/PI3K/AKT signalling pathways, while exerting a protective effect against arterial thrombosis. This study offers new insights into the fields of cardiovascular disease and antiplatelet drug discovery.
Schematic model of AdipoRon regulating platelet activation. (BioRender.com)

Similar content being viewed by others
Introduction
The development of thrombotic disease involves several factors, including vascular endothelial injury, hemodynamic changes, and blood fluid composition changes. The abnormal activation of platelets is a core feature of thrombotic diseases [1]. The pathophysiological changes responsible for the prothrombotic characteristics associated with obesity remain unclear. The adipose tissue secretes several kinds of adipokines in response to various forms of metabolic dysregulation, which may exert cardiovascular effects, thereby contributing to the development of cardiovascular diseases [2,3,4]. Furthermore, clinical studies have shown that obesity is associated with fatal atherosclerotic thrombotic complications, including myocardial infarction and stroke [5]. Adiponectin is an adipocytokine that is abundant in the plasma. However, its level is downregulated in obesity-linked diseases such as coronary artery disease [6, 7]. In addition, serum hypoadiponectinemia is associated with impaired endothelial-dependent vasodilation, hypertension, myocardial infarction, and coronary artery disease [8,9,10,11]. However, the specific link between adiponectin and thrombotic diseases remains unclear.
A previous study showed that adiponectin-knockout mice developed severe diet-induced insulin resistance [12]. Apart from the effects associated with metabolic syndrome, adiponectin deficiency also leads to enhanced thrombus formation and platelet aggregation. The same study described adiponectin as an endogenous antithrombotic factor [13]. Hence, we speculate that the antithrombotic effects of adiponectin are partially associated with the specific interaction of adiponectin with its receptor.
Adiponectin is identified in the human adipose tissue cDNA library, with Acrp30/AdipoQ as its mouse homologue. Adiponectin mRNA was expressed in adipose tissue, and the corresponding protein structure contained two domains: N-terminal collagen-like domain and C-terminal complement component 1q-like globular domain [14]. Adiponectin function is mediated via the adiponectin receptor (AdipoR1 and AdipoR2). Impaired signalling via these receptors may contribute to the development of atherosclerosis [15]. According to studies in vitro and in vivo, adiponectin–adiponectin receptor interactions may be involved in the inhibition of oxidative stress [16, 17], modulation of anti-inflammatory effects [5], vascular protective functions, and anti-apoptotic effects in endothelial cells [18,19,20,21]. However, owing to the difficulty of converting adiponectin into a practical drug form, the adiponectin receptor agonists has been suggested as an alternative [22]. AdipoRon is an orally administered synthetic molecule that can bind to AdipoR1 [23]. In contrast to the well-described intracellular steps and mediators involved in the effect of AdipoRon on insulin sensitivity, the pathways downstream of AdipoRon binding to human platelets remain poorly understood. A lack of systematic analyses on the effects of AdipoRon on platelet activation contributes to the poor understanding of the regulatory role played by adiponectin in haemostatic mechanisms. In this study, we further explored the antithrombotic effects of AdipoRon. We first assessed whether AdipoRon could inhibit platelet activation and then evaluated the intracellular pathways of AdipoRon.
Materials and methods
Ethics approval (animal and human)
All procedures in this study are complied with the guidelines of the European Parliament directive 2010/63/EU on the protection of animals used for scientific purposes. Mice were euthanised by cervical dislocation. Animal experiments were approved by the Institutional Animal Care and Use Committee at Tongji Medical College, Huazhong University of Science and Technology (2020-S2324). Human experiments were approved by the Medical Ethics Committee, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (2020-0245). Informed written consent was obtained before the inclusion of subjects in accordance with the Declaration of Helsinki.
Materials
The following reagents were used in this study: fluorescein isothiocyanate (FITC)-labelled phalloidin, U46619, fibrinogen, and Pluronic F-127 (Sigma-Aldrich; St. Louis, MO, USA); Fluo-3AM (Dojindo Laboratories; Kumamoto, Japan, Lot No. PL657); Collagen and Chrono-lume (Chrono-Log Corporation, Havertown, PA, USA); prostaglandin E1 (PGE), AdipoRon (HY-15848), dorsomorphin (BML-275; Compound C), ADP, recombinant human/mouse adiponectin, emodin, and metformin hydrochloride (MedChem Express; Monmouth Junction, NJ, USA); α-thrombin (Enzyme Research Laboratories; South Bend, IN, USA); Cell Trace Calcein Green (Invitrogen, Carlsbad, CA, USA); FITC-conjugated anti-CD62P (P-selectin) and FITC-conjugated anti-PAC-1 (αIIb3) (Biolegend, San Diego, CA, USA). The primary antibodies were used as follows: p-AMPK (AMPKα, Thr172; #2535), phospho-acetyl-CoA carboxylase (Ser79; #11818), p-p38-mitogen-activated protein kinase (p38-MAPK, Thr180/Tyr182; #4511), p-spleen tyrosine kinase (Syk, Tyr525/526; #2710), p-ERK1/2 (Tyr202/204; #4370), p-PLCγ2 (Tyr759; #3874), p44/42 MAPK (Erk1/2; #4696), and p-protein kinase B (Akt, Ser473; #4060) (Cell Signaling Technology, Danvers, MA, USA); anti-PI3K p85 alpha (phosphoY607; ab182651), and anti-Integrin beta 3 (phospho Y773; ab38460) antibodies (Abcam, Cambridge, UK); AdipoR1 (D-9; sc-518030), p38 alpha/beta MAPK (A-12; sc-7972), Syk (4D10; sc-1240), and PLCγ2 (B-10; sc-5283) antibodies (Santa Cruz Biotechnology, Dallas, TX, USA); ACC1 (67373-1-Ig), Integrin Beta 3 (66952-1-Ig), PI3 Kinase P85 Alpha (60225-1-Ig), AMPK alpha (66536-1-Ig), and AKT (60203-2-Ig) monoclonal antibodies (ProteinTech, Chicago, IL, USA); CKII alpha (Phospho-Tyr255; Biorbyt, UK; orb308948). Tyrode’s buffer comprised the following: 137 mM NaCl, 20 mM HEPES, 13.8 mM NaHCO3, 2.5 mM KCl, 5.5 mM glucose, and 0.36 mM NaH2PO4; pH 7.4.
Human subjects
Recruited healthy volunteers without haematological diseases (like platelet and coagulopathy) had not received any medication in 2 weeks prior to the study.
Animals
C57BL/6 mice (about 8 weeks, weight: 18–22 g) were collected from the Hubei Experimental Animal Research Center. Mice were maintained under standard laboratory condition (55%–65% relative humidity, 22–24 °C, 12-h circadian rhythm, access to food, water).
Preparation of washed human platelets
Peripheral blood was collected from healthy donors and washed platelets were prepared as described previously [24]. After informed consent was obtained, the subject’s blood was collected using a medical silicide vacuumized containers (1:9 (v/v) 3.8% sodium citrate). Whole blood was centrifuged (160 × g, 14 min) to prepare platelet-rich plasma (PRP). After centrifugation at 500 × g for 10 min, the platelets were washed with buffer with prostaglandin E1 (PGE1, 1 μM) and EDTA (2.5 mM). Finally, the platelets were suspended in Tyrode’s buffer without PGE1 or EDTA. Tyrode’s buffer was formulated with 137 mM NaCl, 20 mM HEPES, 13.8 mM NaHCO3, 2.5 mM KCl, 5.5 mM glucose, and 0.36 mM NaH2PO4; pH 7.4. Finally, platelet concentration was adjusted based on experimental requirements, and the samples were allowed to sit for 30 min at 37 °C before each experiment.
Platelet aggregation and adenosine triphosphate (ATP) release assays
Based on previous experiments, we carried out platelet aggregation test and ATP release test [25]. The platelet concentration was adjusted to approximately 300 × 109 cells/L. The washed platelets were pre-incubated with CaCl2 (1 mM), and AdipoRon or solvents at 37 °C for 5 min, then the aggregation was induced by the following reagents: collagen (4.5 µg/mL), thrombin (0.08 U/mL), U46619 (0.12 µg/mL), and CaCl2 (1 mM). PRP was incubated with drugs at 37 °C and stimulated by ADP (1.5 µM). Platelet aggregation was measured by light transmission aggregometry. Platelet ATP release was assessed by adding a luciferase reagent (Chrono-lume) prior to stimulation with agonists (collagen and thrombin). The results were quantified using the aggregation and release plots.
Flow cytometry
The washed human platelets were labelled with FITC-conjugated anti-P-selectin (CD62P) and anti-PAC-1 antibodies before quantitative analysis by flow cytometry. Data were analysed with BD Biosciences (San Jose, CA, USA) flow cytometer.
Calcium signalling
The washed human platelets were labelled with 1 µM Fluo-3AM at 37 °C for 30 min, and then incubated with AdipoRon or vehicle for 5 min. After recording baseline data for 45 s, collagen and 1 mM extracellular calcium were added to fluorescent-activated platelet suspension. The fluorescence intensity was recorded by BD Biosciences flow cytometry.
Measurement of oxygen consumption rate (OCR)
We examined the cellular mitochondrial function using Agilent Seahorse XFe96 (Seahorse Bioscience, North Billerica, MA, USA). The washed human platelets were added into cell-culture microplates for OCR measurement. After monitoring baseline respiration, oligomycin (1 μM), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, 0.5 μM), and rotenone/antimycin (1 μM) were automatically injected into cell-culture microplates to measure the OCR.
Plasmid constructs and transfection
Plasmids were constructed using standard genetic procedures. CKII cDNA was cloned into p-CMV-flag vector. CKII Tyr255 was mutated to alanine based on p-CMV-flag-CKII. According to the company’s instructions, αIIbβ3-CHO cells (supported by the laboratory of Prof Jun-ling Liu, Shanghai Jiao Tong University) were transfected with constructed plasmid, P3000 reagent and Lipofectamine3000 (Invitrogen, CA, USA). αIIbβ3-CHO cells provided a model system for studying the process of αIIbβ3-mediated platelet activation [26]. Cells expressing flag-tagged CKII and flag-tagged CKII-Tyr255A were obtained 48 h after transfection.
Platelet/αIIbβ3-CHO cells spreading on fibrinogen
The washed platelets (18 × 109 cells/L) were incubated with AdipoRon or vehicle, and placed in a 37 °C incubator, to spread on 24-well cell-culture slides. The slides were precoated with fibrinogen (Fg) (25 µg/mL) or BSA, and left overnight at 4 °C. After platelet suspension was removed, the chambers were washed with phosphate-buffered saline (PBS) three times. Adherent platelets were fixed with 2% paraformaldehyde for 10 min, then permeabilised with Triton X-100 (0.1%) and stained with FITC-labelled phalloidin (1:1000). Finally, after washing three times with PBS, the slides were covered on the tissue slides and sealed. Images were taken using a fluorescence microscope (Nikon TE-2000S, Tokyo, Japan). Data were analysed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) to calculate the coverage of spreading platelets.
αIIbβ3-CHO cells were incubated with AdipoRon or vehicle, cultured on cover slips coated with 50 μg/mL Fg for 4 h, then the supernatant was discarded, washed with PBS for three times, and fixed with 4% paraformaldehyde for 10 min. Finally, the cells were stained with FITC-labelled phalloidin and DAPI. Images were obtained using a sectioning scanner (Pannoramic DESK, P-MIDI, P250, 3DHISTECH, Ltd, Budapest, Hungary). Fluorescence was analysed as described for the platelet-spreading experiment.
Clot retraction
Platelet retraction assay was measured in glass tubes (Chrono-Log). The washed platelet suspensions (300 × 109 cells/L) containing 400 μg/mL Fg and 1 mM CaCl2 were pre-incubated with AdipoRon or vehicle. Clot retraction was initiated by thrombin (0.2 U/mL). The platelet suspensions were then incubated at 37 °C. Images were acquired after 30 min. The two-dimensional size of the retracted clots in photographs was quantified using National Institutes of Health ImageJ software.
Flow chamber experiments
Platelet concentration was adjusted and incubated with the fluorescent dye Cell Trace Calcein Green at 37 °C for 30 min, then added to the Bioflux plate (Bioflux 1000Z; Fluxion Biosciences Inc., USA), incubated with vehicle or AdipoRon (10 µg/mL) for 5 min. The Bioflux plate was coated with 100 µg/mL collagen and kept overnight at 4 °C. The labelled platelets were perfused through Bioflux plates at a wall shear rate of 2000/s for 10 min. We measured platelet adhesion using a 10× long-working-distance objective that was utilised for fluorescence and transmitted light microscopy. Platelet activation and aggregation through contact with collagen in the channels was visualised in real time using an inverted epifluorescence microscope (Zeiss Microscope, Shanghai, China). After 10 min, the Bioflux Montage software (Fluxion Biosciences Inc.) was used to assess platelet coverage by intensity and coverage area.
Ferric chloride-induced carotid injury model
Ferric chloride-induced carotid injury model was used to assess the antithrombic activity of AdipoRon. Male C57BL/6 mice were acclimated for at least 1 week before experimentation. Animals were fasted the night before the experiment with free access to water. Mice were injected with AdipoRon (5 or 12.5 mg/kg) or vehicle (10% DMSO + 90% (20% SBE-β-CD in saline)) in the tail vein. The mice were anaesthetised by intraperitoneal injection of 80 mg/kg pentobarbital sodium 30 min later, and the carotid artery was exposed via surgical operation when the degree of anesthesia was appropriate. Doppler flow probe (TS420; Transonic, Ithaca, NY, USA) was placed proximally to the carotid artery to measure basal blood flow. Then, 3-mm strip of filter paper saturated with 12% FeCl3 was pasted on the external surface of carotid artery, timed for 1 min. After the filter paper was removed, carotid blood flow was monitored until stable clots were formed. The occlusion time was defined as the first time point when stable flow interruption was achieved or when 30 min had passed. The changes in blood flow were recorded on the screen. After 8 min, the thrombus was harvested along the carotid vessel, fixed with paraformaldehyde, and embedded in paraffin. Cross sections were stained with haematoxylin and eosin (H&E).
Inferior vena cava (IVC) thrombosis model
Male C57BL/6 mice were prepared as described above. Thirty minutes before surgery, both experimental and control groups were treated with AdipoRon (5 mg/kg) or vehicle, respectively. IVC stenosis was performed as described previously [27]. The mice were anaesthetised with pentobarbital sodium (80 mg/kg) administered via intraperitoneal injection. A midline laparotomy was performed with the abdominal contents removed to one side, and the IVC was separated from the surrounding connective tissue under a stereomicroscope. The side branches between the renal and iliac veins were ligated completely. Finally, the IVC was ligated distal to the renal veins over a 0.3-mm silver wire using a 7–0 polypropylene suture. The wire was subsequently removed, which led to IVC stenosis and marked blood flow restriction. Throughout the surgical procedure, 0.9% sterile saline was applied to prevent drying. After IVC stenosis, 0.2 mL of 1% ampicillin was injected into the abdominal cavity of the mice to prevent infection. The peritoneum was closed using a 6–0 polypropylene suture, and the skin was closed using a 5–0 polypropylene suture. Twenty-four hours after the operation, AdipoRon (5 mg/kg) or vehicle was injected into the tail veins of the experimental or control group, respectively. Venous thrombus formation was observed 48 h after surgery.
Pulmonary thromboembolism model
Mice were injected with either AdipoRon (5 mg/kg) or vehicle in the tail vein. After 30 min, thrombosis was initiated by injecting bovine thrombin (2500 IU/kg, YEASEN Biotech Co., Ltd., Shanghai, China). The behavioural changes and time of death of the mice were recorded. The mice that were still alive after 15 min were euthanised. The lungs were perfused with saline (4 °C), and then fixed with 4% paraformaldehyde for 24 h. The paraffin-embedded lungs were stained with H&E.
Tail bleeding assay
Mice were injected with AdipoRon (5 mg/kg) or vehicle into the tail vein. Thirty minutes later, the tip of mouse tail (1 mm) was subtracted with a scalpel and gently touched on the filter paper at 30 s intervals until the bleeding stopped (with no bleeding spots recorded on the filter paper for three consecutive time points) or until 30 min had passed.
Western blotting
Platelet aggregation reaction products were lysed with a lysis buffer containing a mixture of protease and phosphatase inhibitors. The samples were then boiled at 100 °C for 10 min with loading buffer, separated via 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto a polyvinylidene difluoride membrane (PVDF). The membranes were blocked using 5% BSA in Tris-buffered saline with Tween (TBST) for 1 h at room temperature (RT), then washed thrice with TBST, and blotted with antibodies (1:1000; p-AKT, p-ERK, p-AMPK, p-PLCγ2, p-SYK, p-ACC, p-P38, p-PI3K, p-β3, and p-CKII) overnight at 4 °C. The membranes were then washed with TBST for 3 times, incubated with appropriate secondary antibodies for 1 h at RT. Immunoreactive bands were visualised using the Bio-Rad Gel imaging system (Bio-Rad, Hercules, CA, USA). Protein density was measured using ImageJ software.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad, San Diego, CA, USA). Results are described as mean ± standard error of the mean (SEM). Two-tailed Student’s t tests or one-way analysis of variation (ANOVA) was used to test differences between groups. Statistically significant difference was set at P < 0.05.
Results
Effect of AdipoRon on platelet aggregation
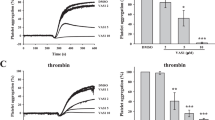
According to statistics, platelet aggregation induced by collagen (4.5 µg/mL), thrombin (0.08 U/mL), U46619 (0.12 µg/mL), and ADP (1.5 µM) was inhibited in a concentration-dependent manner by incubation with AdipoRon for 5 min (Fig. 1a–d). Additionally, AdipoRon inhibited the collagen-stimulated activation of mouse platelets (Supplementary Fig. S1a), but AdipoRon itself could not promote platelet aggregation (Supplementary Figure S1d). We also examined the effects of recombinant human and recombinant mouse adiponectin on platelet activation in humans and mice, respectively, and found that recombinant adiponectin also inhibited platelet activation (Supplementary Fig. S1b, c).
a–c Washed platelets from humans were pretreated with AdipoRon or vehicle for 5 min in the presence of 1 mM CaCl2 and stimulated with collagen (4.5 µg/mL), thrombin (0.08 U/mL), and U46619 (0.12 µg/mL); n = 5. d Platelet-rich plasma from humans was pretreated with either AdipoRon or vehicle and stimulated with ADP (1.5 µM); n = 5. Data are mean ± standard error of mean (SEM); one-way analysis of variance; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS means no significance.
AdipoRon inhibits platelet granule secretion, calcium mobilisation, and mitochondrial respiration
To assess the effects of AdipoRon on platelet granule secretion, the washed platelets were pre-incubated with different concentrations of AdipoRon before stimulation with collagen. The expression of CD62P (P-selectin) was then assessed. Positive platelet P-selectin expression was inhibited by AdipoRon in a dose-dependent manner (Fig. 2a). Further, we analysed the active form of integrin αIIbβ3 by measuring the binding of the monoclonal antibody PAC-1. AdipoRon had an inhibitory effect on collagen-stimulated PAC-1 binding (Fig. 2b). Additionally, AdipoRon inhibited the release of ATP stimulated by collagen and thrombin (Fig. 2c, d). We also measured the effect of AdipoRon on collagen-induced Ca2+ signalling in platelets by flow cytometry. Following collagen stimulation, potent intracellular Ca2+ elevation over time was observed. The signal peaked approximately 160 s after the addition of an agonist. Furthermore, treatment with AdipoRon inhibited Ca2+ response in a dose-dependent manner (Fig. 2e1). Both peak Ca2+ concentration (peak height) (Fig. 2e2) and total influx (AUC) (Fig. 2e3) were reduced. Hence, AdipoRon suppresses intraplatelet calcium mobilisation during platelet activation.
a, b Washed human platelets were stimulated with collagen in the presence of various concentrations of AdipoRon or vehicle at 37 °C and then labelled with fluorescein isothiocyanate-conjugated P-selectin and (FITC)-conjugated PAC-1 antibodies. P-selectin expression (n = 7) and PAC-1 binding (n = 8) were identified by flow cytometry. c, d Washed human platelets were pretreated with various concentrations of AdipoRon or vehicle at 37 °C and then stimulated with collagen/thrombin. The concentration of ATP was assessed using Chrono-lume; n = 5. e1 Collagen (6 µg/mL) induced Ca2+ mobilisation, as determined by the Fluo-3 fluorescent signal and monitored over time by flow cytometry in platelets pretreated with AdipoRon or vehicle. e2 Maximum Ca2+ signal (peak height) and e3 total Ca2+ influx (area under the curve) was quantified using the kinetics curve; n = 5. f The oxygen consumption rate was measured using the Seahorse XF assay. Data are mean ± standard error of mean (SEM); one-way analysis of variance; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS means no significance.
To explore the influence of AdipoRon on mitochondrial respiration, which is important for platelet activation, we measured the OCR. Platelet OCR was measured under the basal condition or with the addition of oligomycin, FCCP, or rotenone/antimycin. The results of the seahorse analysis revealed that ATP-linked respiration, maximal respiration, and spare respiratory capacity declined in the presence of AdipoRon (Fig. 2f), indicating that AdipoRon inhibits mitochondrial respiration.
AdipoRon inhibits platelet integrin αIIbβ3 outside-in signalling in vitro
Platelets exposed to immobilised BSA remained discoid, whereas platelets exposed to immobilised fibrinogen spread to cover a significantly greater surface area. AdipoRon treatment considerably decreased platelet surface area of spread (Fig. 3a). Additionally, the volume of retracted plaque was suppressed in the presence of AdipoRon (2, 4, 10, 20, and 40 µg/mL), compared with that of the control (Fig. 3b). To further confirm the effect of AdipoRon on outside-in signalling, we measured β3 phosphorylation, an indicator of αIIbβ3 activation. Our results suggest that AdipoRon can inhibit the phosphorylation of β3 (Fig. 3c). Moreover, AdipoRon (10 µg/mL) could reduce the adhesion area of platelets to the collagen matrix under arterial shear (Fig. 3d). In addition, we verified that AdipoRon could inhibit the spreading of αIIbβ3-CHO cells (Supplementary Figure S1e).
a Washed human platelets were incubated with various concentrations of AdipoRon or vehicle, exposed to bovine serum albumin (BSA) or immobilised Fg for 60 min. a1 Representative images and a2 summary data of the mean platelet surface area are shown; Scale bar = 20 µm; n = 9. b Washed human platelets were incubated with AdipoRon or vehicle for 120 min and clot retraction assay was performed; n = 5. c Western blotting was performed to assess the phosphorylation levels of integrin β3 (Tyr773); n = 5. d Washed human platelets were labelled with Cell Trace Calcein Green and then incubated with Vehicle or AdipoRon for 5 min. The labelled platelets were perfused through Bioflux plates at a wall shear rate of 2000/s for 10 min. Representative images of surface coverage were revealed; Scale bar = 50 µm; n = 5. Data are mean ± standard error of mean (SEM); one-way analysis of variance (ANOVA); *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS means no significance.
Effect of AdipoRon on carotid artery thrombosis and pulmonary embolism in mice
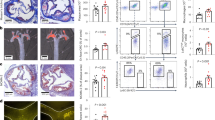
In our study, ferric chloride-induced carotid injury model was used to explore the effect of AdipoRon on arterial thrombosis in vivo. After ferric chloride-induced carotid injury, mice treated with AdipoRon (5 or 12.5 mg/kg) showed prolonged thrombotic occlusion. A longer occlusion time in experimental mice that received 5 mg/kg AdipoRon than in the control group (586.8 ± 43.73 s vs. 242.5 ± 23.09 s). The duration of blood occlusion increased to 1210 ± 142.3 s when AdipoRon concentration was increased to 12.5 mg/kg (Fig. 4a1-2). Histological analysis of the thrombus by H&E staining showed reduced vascular obstruction after 8 min in AdipoRon-treated mice (Fig. 4a3). In the pulmonary thromboembolism model, the death rate was 33% (6/18) in the experimental group and 44% (8/18) in the control group (Fig. 4b1). The mice that received AdipoRon showed fewer pulmonary vessels associated with thrombi than those administered vehicle. Alveolar wall swelling was more severe in the control group (Fig. 4b2).
a1-2 Carotid artery occlusion time was measured after ferric chloride-induced injury using a doppler flow probe. Occlusion time of the AdipoRon (5 mg/kg or 12.5 mg/kg) was compared with that of the vehicle group. a3 Representative histological images of thrombi with haematoxylin and eosin (H&E) staining are shown; Scale bar = 100 µm. b Light microscopic images of H&E-stained lung sections from mice administered normal saline or a mixture of Bovine Thrombin (2500 IU/kg) plus vehicle and Bovine Thrombin (2500 IU/kg) plus AdipoRon (5 mg/kg). The observation time to determine the death and survival of mice was 15 min (AdipoRon group: n = 18 mice; Model group: n = 18 mice). c Venous thrombus formation differed between mice treated with vehicle and those treated with AdipoRon (5 mg/kg) (vehicle, 6 out of 21; AdipoRon 13 out of 21). Furthermore, c2 Weight and c3 length of thrombi were different in the control and experimental groups (Length: AdipoRon 4.269 ± 1.348 mm, n = 13, control 2.583 ± 0.6646 mm, n = 6; P = 0.0105) (Weight: AdipoRon 6.754 ± 3.003 mg, n = 13, control 3.933 ± 1.223 mg, n = 6; P = 0.0427). d Tail bleeding time was determined as the time taken for the initial cessation of bleeding after transection in the normal saline, vehicle, or AdipoRon (5 mg/kg)-treated groups. When the bleeding time was >30 min, it was recorded as 30 min (NS n = 9 mice; vehicle n = 14 mice; AdipoRon n = 14 mice). Data are mean ± standard error of mean (SEM); Unpaired t test; *P < 0.05, **P < 0.01, ****P < 0.0001, NS means no significance.
AdipoRon promotes venous thrombus formation in the IVC stenosis model
The mice were injected with AdipoRon or vehicle before and 24 h after surgery. Venous thrombus formation was observed in 13/21 (62%) mice treated with AdipoRon and in 6/21 (29%) mice in the control group after 48 h of IVC stenosis (Fig. 4c1). Furthermore, the length and weight of the thrombus were different between the AdipoRon- and vehicle-treated mice (length: AdipoRon 4.269 ± 1.348 mm, n = 13; control 2.583 ± 0.6646 mm, n = 6; P = 0.0105; weight: AdipoRon 6.754 ± 3.003 mg, n = 13; control 3.933 ± 1.223 mg, n = 6; P = 0.0427; Fig. 4c2-3).
In terms of haemostatic parameters, there was no significant difference in bleeding time between the mice injected with AdipoRon (5 mg/kg), vehicle, or normal saline (Fig. 4d).
AdipoRon is associated with AMPK phosphorylation in platelets
To explore the mechanism by which AdipoRon inhibits platelet activation, we incubated platelets with varying concentrations of AdipoRon for 5 min and terminated platelet aggregation at the same time. We then isolated protein from the thrombi and conducted Western blotting to confirm that the phosphorylation of the signalling molecule AMPK on Thr172 was inhibited. The phosphorylation of ACC, a molecule downstream of AMPK, was also inhibited (Fig. 5a). In C57BL/6 mice, the AMPK-ACC axis was also inhibited by AdipoRon (Supplementary Figure S2a). To further verify the importance of AMPK in AdipoRon-mediated signalling, we utilised metformin hydrochloride (AMPK agonist) and Compound C (AMPK-specific inhibitor). When incubated along with AdipoRon, metformin hydrochloride prevented the inhibitory effects of AdipoRon, whereas Compound C further aggravated the inhibitory effect of AdipoRon (Supplementary Fig. S2b, c).
a, b Washed human platelets were pretreated with AdipoRon (0, 20, 40 µg/mL) stimulated with collagen and were subsequently lysed with lysis buffer. Western blotting was performed to assess phosphorylation levels of AMPK (Thr172), ACC (Ser79), PLCγ2 (Tyr759), SYK (Tyr525/526), PI3K (Y607), AKT (Ser473), P38, and ERK1/2; n = 5. Data are mean ± standard error of mean (SEM); one-way analysis of variance (ANOVA); *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS means no significance.
AdipoRon inhibits the signalling molecules p-Syk, PLCγ2, phosphoinositide 3-kinase (PI3K), p-p38-mitogen-activated protein kinase (p38-MAPK), and ERK1/2
AdipoRon abolished the phosphorylation of the signalling proteins Syk (Y525/Y526), PLCγ2 (Tyr759), PI3K, and Akt (Ser473) (Fig. 5a). Apart from the AMPK-mediated effects of AdipoRon on Syk/PLCγ2/PI3K/Akt, AdipoRon blocked the phosphorylation of p38-MAPK and ERK1/2 in human platelets (Fig. 5a). Furthermore, pre-incubation with the AMPK inhibitor Compound C showed similar effects to those of AdipoRon and inhibited the phosphorylation of Syk, PLCγ2, PI3K, Akt, p38, and ERK1/2 (Supplementary Fig. S3a, b).
AdipoRon is associated with CKII (Tyr255) phosphorylation in platelets
We next examined whether AdipoRon could inhibit CKIIα phosphorylation (Fig. 6a) and explored the association between CKIIα and the AMPK-ACC axis using a CKIIα inhibitor (Emodin). Inhibition of CKIIα enhanced the phosphorylation of AMPK-ACC (Fig. 6b). In addition, the spreading area of αIIbβ3-CHO cells transfected with the CKII-Tyr255-flag plasmids on fibrinogen was larger than that transfected with the CKII-Tyr255A-flag plasmids (Fig. 6c). Moreover, Compound C inhibited the phosphorylation of CKIIα (Supplementary Figure S3a).
a Washed human platelets were pretreated with AdipoRon (0, 20, 40 µg/mL), stimulated with collagen, and subsequently lysed with lysis buffer. Western blotting was performed to assess phosphorylation levels of CKIIα (Tyr255); n = 5. b Washed platelets were pretreated with Emodin (40 µM/50 µM) or vehicle, stimulated with collagen, and subsequently lysed with lysis buffer followed by immunoblotting using p-ACC (Ser79), p-AMPK (Thr172), and p-CKIIα (Tyr255); n = 5. c Immunofluorescence staining of actin in αIIbβ3-CHO cells transfected with CKII-Tyr255-flag or CKII-Tyr255A-flag plasmids spreading on immobilised fibrinogen; Scale bar = 20 µm; n = 6. Data are mean ± standard error of mean (SEM); one-way analysis of variance (ANOVA); *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS means no significance.
AdipoR1 deficiency impairs the effect of AdipoRon
We next determined that AdipoR1 was expressed in both human and mouse platelets and measured platelet receptor expression levels in AdipoR1-KO (heterozygous) mice (Fig. 7a, b). The platelet count was not significantly different between the KO (heterozygous) and wild-type (WT) mice (Fig. 7c). Moreover, no significant difference was found between AdipoR1-KO (heterozygous) mice and WT platelets in platelet aggregation stimulated by collagen (Fig. 7d). However, AdipoR1 deficiency impaired the inhibitory effect of AdipoRon on platelet aggregation (Fig. 7e).
a AdipoR1 was immunoblotted in human and mouse platelets. b Immunoblotting showed that AdipoR1 expression was decreased in heterozygous-knockout (KO-HE) platelets. c Platelet counts were determined in AdipoR1-KO (HE) and wild-type (WT) mice, and d no significant differences were noted in aggregation levels between KO-HE and WT platelets in response to collagen. n = 5 platelets from 4 mice. e Aggregation was measured in AdipoR1-KO (HE) platelets in the presence of AdipoRon, with collagen stimulation. n = 5 platelets from 4 mice. Data are mean ± standard error of mean (SEM); t-tests; *P < 0.05, **P < 0.01, NS means no significance.
Discussion
Adipose tissue produces numerous bioactive substances known as adipokines, including leptin, resistin, and adiponectin [28]. Unlike other adipokines, adiponectin plays a protective role against the development of atherosclerotic vascular disease [29, 30]. This might be because adiponectin decreases endothelial damage and stimulates the production of NO from vascular endothelial cells [23]. However, the exact mechanism of this protective effect on platelets exerted by adiponectin in thrombotic disease remains unknown. AdipoRon is an orally administered synthetic molecule that can bind to AdipoR1/2 [31] and produces the same effects in platelets as adiponectin by inhibiting their aggregation, which was confirmed in our study. A previous study showed that adiponectin inhibits platelet aggregation in response to agonists such as ADP and epinephrine [32]. Our study confirmed that AdipoRon could also inhibit platelet aggregation excited by ADP, thrombin, collagen, and U46619, which not only confirmed the findings of previous studies, but also provided additional insights. Here, we investigated the mechanism of AdipoRon to gain insights into how adiponectin receptors are associated with cardiovascular diseases via the inhibition of platelet activation.
Platelet granules contain abundant proteins such as P-selectin, fibrinogen, and von Willebrand factor. Dense granules are rich in ADP, ATP, and 5-hydroxytryptamine. Degranulation of platelets enables the release of these substances to further promote the haemostatic effect of platelets [33]. When platelets are activated, a variety of coagulation factors and platelet-activating factors are usually released by secretory granules, and the process is known as degranulation [34]. Our study verified the release of these factors stimulated by collagen, and further revealed that AdipoRon can inhibit the redistribution of P-selectin and the secretion of dense granules (ATP).
Platelet activation involves drastic structural changes that require high energy and fully functional mitochondria [35]. The production of mitochondrial ATP is important in order to meet the increased energy requirements of activated platelets [36]. Therefore, mitochondrial activity, as represented by mitochondrial OCRs, has been reported to be closely related to the level of platelet aggregation [37]. Previous studies have shown that AMPK activity is closely linked to mitochondrial respiration [38]. Accumulating studies have demonstrated crosstalk between AMPK and mitochondria. AMPK promotes mitochondrial biogenesis in a variety of tissues, including myocytes, adipocytes, and hepatocytes [39]. In the current study, AdipoRon was found to inhibit AMPK phosphorylation. We suspected that mitochondrial respiration is inextricably related to AdipoRon regulating platelet function in platelets. Therefore, we speculated whether AdipoRon could inhibit platelet mitochondrial energy metabolism. We measured the OCR and found that AdipoRon did not affect platelet basal oxygen consumption but instead reduced maximal respiration in the OCR, indicating that AdipoRon may affect platelet bioenergetics. According to our findings, the AMPK-ACC signalling pathway can be inhibited by AdipoRon. However, Lepropre et al. previously demonstrated that AMPK-induced ACC1 phosphorylation in platelets does not play a major role in mitochondrial respiration [40]. Therefore, the association between AMPK and mitochondrial respiration in platelets deserves further exploration, which is of great significance for the study of energy metabolism and activity of platelets.
The binding of platelets to fibrinogen, which is critical for firm adhesion and thrombus formation, relies on conformational changes of integrin αIIbβ3 in response to agonists [41]. The inhibition of AdipoRon on αIIbβ3 signalling was confirmed in the current study by platelet-spreading and clot retraction assays, as well as β3 phosphorylation measurement.
An increased platelet aggregation response was found in adiponectin deficient mice, and this phenotype was inhibited when recombinant adiponectin was added, suggesting a link between adipose tissue factor and thrombosis [42]. Platelets are important in the development of potential cardiovascular disease. However, to date, there is no evidence of the function of adiponectin receptors in platelets. Previous studies have shown that human and mouse AdipoR1 share 96.8% identity [43]. Therefore, we extended these in vitro findings by analysing arterial thrombosis in vivo using a ferric chloride-induced carotid artery injury mice model and found that AdipoRon inhibits arterial thrombosis in vivo. Interestingly, our results indicate that AdipoRon could promote the formation of venous thrombosis in vivo. In addition, the length and weight of thrombi formed in AdipoRon-stimulated mice were larger than those in the control group. Our results do not support the hypothesis that AdipoRon exerts an antithrombotic effect on venous thrombus formation in mice. The opposing results of this study may be due to the different pathophysiological mechanisms between arterial thromboses and venous thromboses. That is, the thrombus in venous thrombosis is rich in fibrin and red blood cells, while the clot component in arterial thrombosis is platelet-rich thrombus [44]. The main causes of deep vein thrombosis are changes in blood composition, reduced blood flow, and damage to blood vessel wall. Additionally, we employed a vessel blood flow restriction model of venous thrombosis. After flow reduction, the number of recruited leucocytes clearly outnumbered the platelets accumulated. Venous thrombus embolism was associated with increased production of multiple chemokines, such as CCL2, CXCL1, and CXCL5, which contribute to the transport of leucocyte to the endothelial surface. Furthermore, expression of endothelial P-selectin was found to be essential for the accumulation of leucocytes on the surface of endothelial cells. Therefore, endothelial P-selectin is very important for deep venous thrombosis, while platelet P-selectin is relatively less important for venous thrombosis. These findings indicate that platelet aggregation does not play a crucial role in the initiation of venous thrombosis [45]. Alternatively, we used the FeCl3-induced vascular injury model to induce arterial thrombi. Studies have shown that when FeCl3 is attached to the carotid artery, tissue factors are exposed to the surface of FeCl3-containing microspheres, promoting thrombin production and causing platelet activation, adhesion and coagulation, suggesting the importance of platelet activation in arterial thrombosis [46]. In addition, the main adverse effect of current antiplatelet drugs is bleeding, which limits their use [44]. We demonstrated that AdipoRon has no effect on mouse tail bleeding time, which indicates that drugs like AdipoRon are safe to use as antithrombotic agents.
The seven-transmembrane helices of AdipoR1 and AdipoR2 are conformably different from the GPCRs and encase a large void in which three conserved histidine residues are coordinated with a zinc ion [23]. The crystal structures of these receptors may provide a strong basis for the development and optimisation of small-molecule compounds. like AdipoRon. The effects of AdipoRon in cardiac myocytes have been partially attributed to AMPK activation [31]. However, the effect of AdipoRon on platelet function and related pathways remains unclear, and this study showed that by binding to human platelets, AdipoRon inhibited p-AMPK (Thr172) upon stimulation with collagen. This was further confirmed using metformin hydrochloride, an AMPK agonist. Therefore, the role of AdipoRon in platelets appears to differ from that in other cells, and AdipoRon signal transduction is consistent with the glycoprotein VI (GPVI) signalling pathway. Furthermore, this finding indicated that the inhibition site of AdipoRon was located upstream of the signalling pathway of platelet aggregation induced by collagen stimulation. We also used AdipoR1-KO (heterozygous) mice to further validate the effects of AdipoRon on platelets. Our results showed that the loss of the AdipoR1 receptor did not affect platelet formation, basal activity, and downstream GPVI signalling. However, the lack of AdipoR1 alleviated the inhibitory effect of AdipoRon, indicating that AdipoRon may affect the downstream pathway through AdipoR1 on platelets and may ultimately inhibit the energy metabolism and collagen-mediated downstream signalling of GPVI, which provides an important entry point for the AdipoRon signalling pathway in platelets. This may provide a new target in the study of antiplatelet drugs.
The primary platelet-specific receptor GPVI can bind to collagen to initiate platelet aggregation by recognising VWF/collagen in the damaged blood vessel wall [47]. Activation of αIIbβ3 outside-in signalling by ligand binding could induce tyrosine phosphorylation of signal proteins [48, 49] including Syk, c-Src, and PLCγ2, which ultimately promotes downstream platelet responses, such as granule secretion and platelet spreading [50, 51]. PI3K is also activated by collagen-GPVI [52]. This study revealed that AdipoRon inhibits the phosphorylation of PLCγ2, Syk, PI3K, and Akt (the primary downstream effector of PI3K) in human platelets, an effect mediated by AMPK, indicating that the AMPK signalling pathway resides upstream of these signals.
Platelets contain ERK1/2 and p38-MAPK, which are members of the MAPK family [53]. MAPKs in platelets have long been considered important for cytosolic phospholipase A2 activation and thromboxane A2 generation [54]. However, the specific ERK1/2 pathways in platelet activation remain unclear. In the present study, AdipoRon effectively inhibited collagen-induced platelet aggregation via the suppression of p38-MAPK and ERK1/2 phosphorylation. We further verified that p38-MAPK and ERK1/2 are both biologically important signalling molecules that act in response to AdipoRon.
Casein kinase 2, as a serine/threonine protein kinase, has a tetramer structure including two catalytic and two regulatory subunits. Ryu et al. demonstrated that CK2 plays a key role in platelet aggregation, secretion, TxA2 generation, possibly through regulation of PI3K pathway and phosphorylation of Akt and ERK. CKII is also involved in αIIbβ3 outside-in signalling in platelets as reduced platelet spreading and clot reaction could be observed after CK2 inhibition [55]. Moreover, previous studies have investigated the β subunit of CKII; deficiency of the platelet CKIIβ receptor leads to the abrogation of alpha and dense granules secretion of platelets and impairs the activation and aggregation function of integrin αIIbβ3, which may be due to the disruption of tubulin aggregation in CKIIβ-/- platelets [56]. However, research on the catalytic subunit of CKII in platelets remains unclear. Tyr255 is reported to be responsible for the increased catalytic activity induced by CKIIα tyrosine phosphorylation, but its specific role remains unclear [57]. In the current study, we found that phosphorylation of CKII Tyr255 increases during platelet activation, and AdipoRon inhibits the increase in CKII phosphorylation. According to our results, AMPK phosphorylation was enhanced when CKII was inhibited, indicating that CKII might be downstream of AMPK and may thus control the signal transduction of platelet activation. Furthermore, the phosphorylation of CKII Tyr255 was found to be significantly associated with integrin signalling, which is of great significance for revealing novel signalling pathways in platelets and for selecting specific sites for the development of antiplatelet drugs.
The present study shows that AdipoRon inhibits αIIbβ3 activation, calcium mobilisation, and cytoskeletal reorganisation in platelets. AdipoRon binding affects AMPK phosphorylation and CKII phosphorylation, as well as further downstream molecules (Syk, PLCγ2). This appears to involve the signalling molecule PI3K, which may modulate platelet activation via Akt. We also showed that p38-MAPK and ERK1/2 participate in intracellular signalling pathways in platelets inhibited by AdipoRon. These results improve our understanding of the association between hypoadiponectinemia and increased risk of obesity-related cardiovascular disease, and provide new insights into the development of antiplatelet drugs.
References
Gregg D, Goldschmidt-Clermont PJ. Cardiology patient page. Platelets and cardiovascular disease. Circulation. 2003;108:e88–90.
Maenhaut N, Van de Voorde J. Regulation of vascular tone by adipocytes. BMC Med. 2011;9:25.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97.
Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014;233:104–12.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88.
Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–6.
Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89.
Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–4.
Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7.
Kiris I, Tekin I, Yesildag A, Vural H, Oyar O, Sirin B, et al. Inverse relationship between adiponectin levels and subclinical carotid atherosclerosis in patients undergoing coronary artery bypass grafting. Int Heart J. 2006;47:855–66.
Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH, et al. Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension. 2007;49:1455–61.
Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7.
Kato H, Kashiwagi H, Shiraga M, Tadokoro S, Kamae T, Ujiie H, et al. Adiponectin acts as an endogenous antithrombotic factor. Arterioscler Thromb Vasc Biol. 2006;26:224–30.
Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33.
Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab. 2014;28:15–23.
Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9.
Matsuda M, Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev Endocr Metab Disord. 2014;15:1–10.
Kockx MM, Herman AG. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc Res. 2000;45:736–46.
Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–6.
Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–31.
Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, et al. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–94.
Lee CH, Hung YJ. Possible new therapeutic approach for obesity-related diseases: role of adiponectin receptor agonists. J Diabetes Investig. 2015;6:264–6.
Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–9.
Cheng Z, Gao W, Fan X, Chen X, Mei H, Liu J, et al. Extracellular signal-regulated kinase 5 associates with casein kinase II to regulate GPIb-IX-mediated platelet activation via the PTEN/PI3K/Akt pathway. J Thromb Haemost. 2017;15:1679–88.
Miao S, Shu D, Zhu Y, Lu M, Zhang Q, Pei Y, et al. Cancer cell-derived immunoglobulin G activates platelets by binding to platelet FcγRIIa. Cell Death Dis. 2019;10:87.
Xu Y, Jiang H, Li L, Chen F, Liu Y, Zhou M, et al. Branched-chain amino acid catabolism promotes thrombosis risk by enhancing tropomodulin-3 propionylation in platelets. Circulation. 2020;142:49–64.
Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Köllnberger M, et al. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–7.
Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1–E19.
Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–91.
Zoccali C, Mallamaci F, Tripepi G, Benedetto FA, Cutrupi S, Parlongo S, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–41.
Zhang Y, Zhao J, Li R, Lau WB, Yuan YX, Liang B, et al. AdipoRon, the first orally active adiponectin receptor activator, attenuates postischemic myocardial apoptosis through both AMPK-mediated and AMPK-independent signalings. Am J Physiol Endocrinol Metab. 2015;309:E275–282.
Restituto P, Colina I, Varo JJ, Varo N. Adiponectin diminishes platelet aggregation and sCD40L release. Potential role in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2010;298:E1072–1077.
Heijnen H, van der Sluijs P. Platelet secretory behaviour: as diverse as the granules … or not? J Thromb Haemost. 2015;13:2141–51.
Yeh JJ, Tsai S, Wu DC, Wu JY, Liu TC, Chen A. P-selectin-dependent platelet aggregation and apoptosis may explain the decrease in platelet count during Helicobacter pylori infection. Blood. 2010;115:4247–53.
Schoenwaelder SM, Darbousset R, Cranmer SL, Ramshaw HS, Orive SL, Sturgeon S, et al. 14-3-3ζ regulates the mitochondrial respiratory reserve linked to platelet phosphatidylserine exposure and procoagulant function. Nat Commun. 2016;7:12862.
Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–10.
Zhang W, Ma Q, Siraj S, Ney PA, Liu J, Liao X, et al. Nix-mediated mitophagy regulates platelet activation and life span. Blood Adv. 2019;3:2342–54.
Wang Y, An H, Liu T, Qin C, Sesaki H, Guo S, et al. Metformin improves mitochondrial respiratory activity through activation of AMPK. Cell Rep. 2019;29:1511–23.
Wu S, Zou MH. AMPK, mitochondrial function, and cardiovascular disease. Int J Mol Sci. 2020;21:4987.
Lepropre S, Kautbally S, Octave M, Ginion A, Onselaer MB, Steinberg GR, et al. AMPK-ACC signaling modulates platelet phospholipids and potentiates thrombus formation. Blood. 2018;132:1180–92.
Ya F, Xu XR, Shi Y, Gallant RC, Song F, Zuo X, et al. Coenzyme Q10 upregulates platelet cAMP/PKA pathway and attenuates integrin αIIbβ3 signaling and thrombus growth. Mol Nutr Food Res. 2019;63:e1900662.
Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–70.
Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9.
Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–8.
von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–35.
Eckly A, Hechler B, Freund M, Zerr M, Cazenave JP, Lanza F, et al. Mechanisms underlying FeCl3-induced arterial thrombosis. J Thromb Haemost. 2011;9:779–89.
Qiao J, Arthur JF, Gardiner EE, Andrews RK, Zeng L, Xu K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol. 2018;14:126–30.
Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–9.
Clark EA, Shattil SJ, Ginsberg MH, Bolen J, Brugge JS. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin alpha IIb beta 3. J Biol Chem. 1994;269:28859–64.
Phillips DR, Jennings LK, Edwards HH. Identification of membrane proteins mediating the interaction of human platelets. J Cell Biol. 1980;86:77–86.
Shattil SJ, Kashiwagi H, Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–57.
Watson SP, Herbert JM, Pollitt AY. GPVI and CLEC-2 in hemostasis and vascular integrity. J Thromb Haemost. 2010;8:1456–67.
Börsch-Haubold AG, Ghomashchi F, Pasquet S, Goedert M, Cohen P, Gelb MH, et al. Phosphorylation of cytosolic phospholipase A2 in platelets is mediated by multiple stress-activated protein kinase pathways. Eur J Biochem. 1999;265:195–203.
Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–78.
Ryu SY, Kim S. Evaluation of CK2 inhibitor (E)-3-(2,3,4,5-tetrabromophenyl)acrylic acid (TBCA) in regulation of platelet function. Eur J Pharmacol. 2013;720:391–400.
Münzer P, Walker-Allgaier B, Geue S, Langhauser F, Geuss E, Stegner D, et al. CK2β regulates thrombopoiesis and Ca2+-triggered platelet activation in arterial thrombosis. Blood. 2017;130:2774–85.
Donella-Deana A, Cesaro L, Sarno S, Ruzzene M, Brunati AM, Marin O, et al. Tyrosine phosphorylation of protein kinase CK2 by Src-related tyrosine kinases correlates with increased catalytic activity. Biochem J. 2003;372:841–9.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 31620103909 to YH and No. 81800134 to ZPC) and the Fundamental Research Funds for the Central Universities (HUST: 2021yjsCXCY122) to XHZ. We would like to thank Prof Jun-ling Liu’s team from the Department of Biochemistry and Molecular Cell Biology, Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Shanghai Jiao Tong University School of Medicine, for providing αIIbβ3-CHO cells for our experiment.
Author information
Authors and Affiliations
Contributions
YH designed the study. XHZ, ZPC conducted the experiments and analysed data. XHZ, ZPC and YH drafted the manuscript. ML, WYL, LLL and ZYM helped perform the experiments. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Xh., Cheng, Zp., Lu, M. et al. Adiponectin receptor agonist AdipoRon modulates human and mouse platelet function. Acta Pharmacol Sin 44, 356–366 (2023). https://doi.org/10.1038/s41401-022-00943-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-00943-1
Keywords
This article is cited by
-
Exploring the logic and conducting a comprehensive evaluation of AdipoRon-based adiponectin replacement therapy against hormone-related cancers—a systematic review
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Progress of nanomaterials in the treatment of thrombus
Drug Delivery and Translational Research (2024)