Abstract
Additional therapeutic options are needed for relapsed and refractory multiple myeloma (RRMM). We present data from a phase 1b, open-label, dose-escalation study (NCT01965353) of 20 patients with RRMM (median age: 63 years [range: 50–77]) and a median of four prior regimens (range: 2–14); 85% had refractory disease (lenalidomide [80%]; bortezomib [75%]; lenalidomide and bortezomib [50%]). Patients received a median of six cycles (range: 1–74) of panobinostat (10 or 15 mg), lenalidomide 15 mg, bortezomib 1 mg/m2, and dexamethasone 20 mg (pano-RVd). Median follow-up was ~14 months. Six dose-limiting toxicities were reported (mostly hematological); maximum tolerated dose of panobinostat (primary endpoint) was 10 mg. Most common adverse events (AEs) were diarrhea (60%) and peripheral neuropathy (60%); all grade 1/2. Grade 3/4 AEs occurred in 80% of patients and included decreased neutrophil (45%), platelet (25%) and white blood cell (25%) counts, anemia (25%) and hypophosphatemia (25%). No treatment-related discontinuations or mortality occurred. In evaluable patients (n = 18), overall response rate was 44%, and clinical benefit rate was 61%. Median duration of response was 9.2 months; progression-free survival was 7.4 months; overall survival was not reached. Pano-RVd proved generally well-tolerated and demonstrated potential to overcome lenalidomide and/or bortezomib resistance.
Similar content being viewed by others
Introduction
Treatment of multiple myeloma (MM), an incurable plasma-cell neoplasm, has changed substantially in recent decades1. Rapidly evolving treatment standards have led to improvements in overall survival (OS), as well as the depth and duration of response (DOR)2,3,4. Nevertheless, most patients ultimately relapse and require subsequent lines of therapy, with the depth of response and DOR to each successive regimen typically decreasing over time1. However, different emerging classes of agents, which can be combined in triplet or even quadruplet regimens, have provided clinicians with several new therapeutic options for relapsed and refractory MM (RRMM) patients.
Histone deacetylase inhibitors (HDACis), which influence transcriptional activation and other nuclear events by increasing histone acetylation, have been developed in recent years as a treatment for RRMM1,5,6,7. Histone deacetylases (HDACs) are overexpressed in MM, leading to reduced expression of tumor suppressor genes and, consequently, increased growth and proliferation of tumor cells. Unsurprisingly, overexpression of class I HDACs is associated with a poorer prognosis in MM8.
Broad-spectrum HDACis can enhance the anti-MM activity of proteasome inhibitors through multiple non-mutually exclusive mechanisms, including the transcriptional repression of ubiquitin/proteasome pathway genes and drivers of tumor cell survival and treatment resistance9,10, as well as the inhibition of the aggresome, which is an alternative route for protein degradation11. Panobinostat is among the most potent HDACis in clinical development and the only HDACi approved for the treatment of RRMM, in combination with bortezomib and dexamethasone12,13. In the phase 2 PANORAMA 2 trial, heavily pre-treated, bortezomib-refractory patients with RRMM had an overall response rate (ORR) of 34.5% with panobinostat plus bortezomib and dexamethasone (pano-Vd), highlighting that the addition of panobinostat can overcome resistance to prior therapeutic agents, including bortezomib14. In the randomized phase 3 PANORAMA 1 study, pano-Vd significantly improved median progression-free survival (PFS) by 12.5 vs. 4.7 months (hazard ratio 0.47; 95% confidence interval [CI] 0.31, 0.72) in patients who had received ≥2 prior regimens including bortezomib and an immunomodulatory drug (IMiD), and nearly tripled the rate of complete response/near complete response, compared with placebo-Vd (22% vs. 8% for pano-Vd and placebo-Vd, respectively)5,6,15.
The optimal dose and schedule of pano-Vd was confirmed in the randomized phase 3 PANORAMA 3 trial as 20 mg thrice weekly; DOR with this regimen was 22.6 months and tolerability was improved with subcutaneous administration of bortezomib with only 11.5% of patients in the 20 mg TIW dosing group reporting grade 3/4 diarrhea compared with intravenous delivery of bortezomib, as was standard practice at the time of previous clinical trials.16
Broad-spectrum HDACis also enhance the anti-MM activity of IMiDs, such as lenalidomide, by suppressing diverse oncogenic transcriptional programs10, including the interferon regulatory factor-4/MYC axis17. In a phase 2 study of patients with RRMM, panobinostat plus lenalidomide and dexamethasone (pano-Rd) demonstrated an encouraging ORR (41%) and median PFS (7.1 months) in patients with high-risk, lenalidomide-refractory (81%), and/or bortezomib-refractory (52%) MM, suggesting that panobinostat is also able to overcome resistance to IMiDs.
These data provided the rationale for investigating the quadruplet regimen, panobinostat, lenalidomide, bortezomib and dexamethasone (pano-RVd), in heavily pre-treated patients, particularly in those refractory to proteasome inhibitors and/or IMiDs for whom additional therapy options are needed. Here, we report the findings of a phase 1b dose-escalation study of pano-RVd with extended follow-up.
Methods
Study design and objectives
This study was an open-label, multicenter, phase 1b, study of pano-RVd in RRMM (NCT01965353). Primary objectives were to identify the maximum tolerated dose (MTD) of panobinostat in combination with RVd and to evaluate the safety profile of pano-RVd. Secondary objectives were to evaluate ORR, DOR, time to progression (TTP), PFS and OS. A modified Fibonacci design was used, with 3–6 patients planned at each dose level followed by a dose-expansion phase with an additional ten patients to evaluate the tolerability of the MTD.
The study was conducted in accordance with the Declaration of Helsinki, US Code of Federal Regulations governing clinical study conduct, state laws and Dana-Farber/Harvard Cancer Center research policies and procedures. The institutional review board for each center approved the protocol and amendments. All patients provided written informed consent before enrollment.
Patient eligibility
Eligible patients were aged ≥18 years with measurable RRMM (2011 International Myeloma Working Group consensus)18, an Eastern Cooperative Oncology Group performance status <2, and had received ≥2 lines of therapy. Patients with primary refractory disease, prior HDACi treatment, creatinine clearance <45 mL/min, platelet count <75,000 cells/mm3, absolute neutrophil count <1500 cells/mm3, or hemoglobin level <8.0 g/dl at screening were excluded. Patients with ≥grade (G) 2 peripheral neuropathy (PN) or hepatic impairment (bilirubin > 1.5 × institutional upper limit of normal, or aspartate aminotransferase, alanine aminotransferase, or alkaline phosphatase >2 × institutional upper limit of normal) within 21 days of study therapy initiation were also excluded.
Treatments administered
Patients received oral panobinostat 10 mg or 15 mg plus subcutaneous bortezomib 1 mg/m2, oral lenalidomide 15 mg, and oral dexamethasone 20 mg in 21-day cycles per the schema in Fig. 1. After eight cycles, patients switched to a maintenance schedule, with reduced bortezomib and dexamethasone dosing. Treatment continued until disease progression, unacceptable toxicity, consent was withdrawn, or discontinuation was in the best interest of the patient. Doses could be held for up to 21 days or reduced to manage therapy-related adverse events (TRAEs).
Necessary concomitant medications were allowed, except for those which may cause QTcF prolongation or induce torsades de pointes. Leukocyte growth factors were only administered during cycle 1 in the event of a dose-limiting toxicity (DLT) (if appropriate), but could be prescribed for severe neutropenia after cycle 1.
Dose escalation, MTD, and DLTs
A standard 3 + 3 dose-escalation schema was used (Supplementary Fig. S1). If ≥2 of 3 patients within a cohort experienced a DLT during the dose-escalation phase, the dose immediately below the current dose was defined as the MTD. The 3 + 3 schema was chosen, rather than an alternative methodologic phase 1 study design, due to the limited number of patients in the study and the fact that only three dose levels were planned. DLTs were assessed during the first cycle and defined as a QTcF interval >500 ms, or an absolute increase of >60 ms, a ≥ G3 non-hematological AE or a G4 hematological AE (including thrombocytopenia with platelets <25,000/mm3 on >1 occasion, or G4 neutropenia for >5 days and/or resulting in neutropenic fever on two occasions). Lymphopenia, an AE associated with bortezomib use, was not considered a DLT. Inability to take ≥75% of the planned study drug doses, or receive day 1 doses for cycle 2 due to a drug-related AE occurring in cycle 1, were also considered DLTs.
Assessments
AEs, graded using Cancer Therapy Evaluation Program Version 4 of the National Cancer Institute Common Terminology Criteria for Adverse Events19, were assessed throughout the study and for 30 days after completion of study therapy. Patients who discontinued for any reason other than disease progression were followed every three months until disease progression. Disease response/progression were assessed at the start of each treatment and maintenance cycle, at the end of study, and during the follow-up phase. Disease response was assessed locally using the International Myeloma Working Group Response Criteria18 with M-protein quantification and immunofixation in serum and 24-h urine samples, and serum-free light chain testing. OS was assessed every three months after disease progression.
Statistical analyses
Data cut-off was 24 January 2019. Descriptive statistics are provided for the reported outcomes. All participants who had received ≥1 dose of any study treatment were evaluated for AEs from treatment initiation. All patients who had received study treatment and had ≥1 follow-up assessment were included in the response evaluation. Only patients who responded to treatment (≥minimal response) were included in the DOR analysis. DOR, TTP, PFS, and OS were estimated using Kaplan–Meier methodology. DOR was measured as the time from initiation of first response to time of disease progression, death, or last follow-up (for those patients who had not progressed or died). TTP was defined as time from registration to progression or to last follow-up (for those who had not progressed). PFS was defined as the time from registration to disease progression, death, or last follow-up (for those who had not progressed or died). OS was defined as time from registration to death or last follow-up (for patients who had not died).
Results
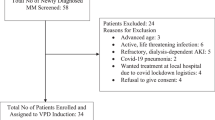
Patient characteristics
Between November 2013 and October 2016, 20 patients were enrolled: median age was 63 years (range: 50–77), 70% male (Table 1). Cytogenetic data were available for 17 patients; two had t(4;14), and one had t(14;16). The median number of prior lines of treatment was four (range: 2–14); five patients had received >5 prior lines of treatment. Overall, 95% and 90% had been previously treated with bortezomib and lenalidomide, respectively. Most patients (n = 17, 85%) had refractory disease (lenalidomide [80%]; bortezomib [75%]; lenalidomide and bortezomib [50%]; dexamethasone [85%]).
MTD and DLTs
Three patients were treated with panobinostat 10 mg; one experienced a DLT; per protocol, three additional patients were treated with panobinostat 10 mg. No further DLTs were reported, and the dose was escalated. Three patients were treated with panobinostat 15 mg; two experienced DLTs. One of these DLTs (syncope) was a pre-existing condition and not considered related to study treatment, thus, one additional patient was treated with panobinostat 15 mg; this patient subsequently experienced a DLT (thrombocytopenia). Therefore, 10 mg was defined as the MTD for panobinostat in the pano-RVd combination. In the expansion phase, ten patients were treated with pano-RVd with panobinostat dosed at 10 mg; two experienced a DLT. Overall, four patients experienced one DLT, one patient experienced two DLTs and one patient experienced three DLTs (Table 2).
Treatment summary
Patients completed a median of six cycles (range: 1–74) (Supplementary Table S1). Three patients completed one cycle before withdrawing due to progressive disease. Eight patients completed all eight treatment cycles and ≥1 cycle of maintenance therapy. At the data cut-off date, one patient remained on treatment (Supplementary Fig. S2).
Safety and tolerability
All 20 patients experienced ≥1 TRAE. The distribution of worst overall TRAEs experienced was G1 (n = 1), G2 (n = 3), G3 (n = 11) or G4 (n = 5). The most common TRAEs included diarrhea (60%, all were G1/2), PN (60%), anemia (55%), fatigue (55%), neutropenia (55%), constipation (50%) and hypokalemia (50%) (Table 3). The median (95% CI) duration of neutropenia and thrombocytopenia AEs was 7 (4; 11) days and 8 (4; 11) days, respectively. Overall, 37 G3/4 TRAEs were reported; the most common were decreased neutrophil (45%), platelet (25%) and white blood cell (25%) counts; anemia (25%); and hypophosphatemia (25%). The rate of cardiac TRAEs was low (atrial fibrillation, n = 1; atrial flutter, n = 1; sinus bradycardia, n = 1; sinus tachycardia, n = 1; palpitations, n = 1; prolongation of QTcF interval, n = 1; heart racing, n = 1; chronic right bundle branch block, n = 1; ectopy and murmur n = 1), and all events were G1/2. No G3/4 diarrhea TRAEs were observed. The only reported G4 TRAEs were decreased platelet (25%) and white blood cell counts (5%). In total, nine serious AEs were experienced by six patients, including treatment-related hyperglycemia, decreased neutrophil count, fatigue, and decreased platelet count. TRAE rates were generally balanced in patients in patients <65 (n = 11) and ≥65 (n = 9) years old, although G4 thrombocytopenia TRAEs were more common in patients ≥65 years old.
There were no instances of treatment-related death or study discontinuation. Dose levels of lenalidomide, bortezomib, dexamethasone, and panobinostat were reduced in at least one cycle for three, six, six, and three patients, respectively. Dose reductions for panobinostat and lenalidomide only occurred in patients in the panobinostat 15 mg cohort. At least one cycle of the pano-RVd regimen was delayed in 18 patients. The most common reason for dose holds was AEs (73%), of which upper respiratory infection, decreased neutrophil count and peripheral sensory neuropathy were the most common (Supplementary Table S2). Reasons for treatment withdrawal included progressive disease (85%), death (5%) and autologous stem cell transplantation (5%).
Responses and outcomes
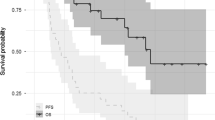
The median follow-up was ~14 months. Per protocol, two patients were not eligible for response evaluation; one died due to factors unrelated to study treatment 2 weeks after starting study treatment, and one withdrew from the study 3 days after starting treatment, due to rapid disease progression. In response-evaluable patients (n = 18), the rate of very good partial response or better was 17%, the ORR (≥partial response) was 44% (90% CI 24, 66) and the clinical benefit response rate (≥minimal response) was 61% (90% CI 39, 80) (Table 4). In patients refractory to both lenalidomide and bortezomib (n = 10), the ORR was 30% (90% CI 9, 61) and the clinical benefit rate was 50% (90% CI 22, 78). Median time-to-first response was approximately 1 month (range: 0.79–4.6), and the median DOR was 9.2 months (range: 2.1–50.4 months). At data cut-off, four patients had a DOR of at least 10 months, median (95% CI) TTP was 7.8 (7.2, not reached) months, median (95% CI) PFS was 7.4 (4.2, not reached) months (Fig. 2), and median (95% CI) OS had not been reached (13.5, not reached). In patients refractory to both lenalidomide and bortezomib, median (95% CI) DOR was 22.1 (9.2, not reached) months, median (95% CI) TTP and PFS were both 22.9 (0.9, not reached) months (Fig. 2), and median (95% CI) OS was not reached (6.8, not reached).
Discussion
Despite recent approvals of new therapeutic agents for RRMM, nearly all patients ultimately still experience disease relapse. The pano-RVd regimen investigated in this study provides a potential treatment option that can restore responses in heavily treated patients with refractory disease. The MTD of panobinostat in the pano-RVd regimen was 10 mg, dosed in a 2-weeks-on/1-week-off schedule, in combination with bortezomib 1 mg/m2, lenalidomide 15 mg and dexamethasone 20 mg. Although all patients experienced at least one TRAE, no patients discontinued therapy or were withdrawn from the study due to a TRAE. Importantly, this regimen demonstrated promising activity in RRMM patients, including those who were refractory to bortezomib and/or lenalidomide and had received a median of four prior lines of therapy. Furthermore, DOR, TTP, and PFS efficacy outcomes were favorable, even in patients refractory to both lenalidomide and bortezomib.
Most patients in the study were refractory to bortezomib or lenalidomide (75% and 80%, respectively) and 50% were refractory to both bortezomib and lenalidomide. The overall ORR of 44% and clinical benefit rate of 61% demonstrates that pano-RVd can provide disease control in patients previously treated with and resistant to lenalidomide and/or bortezomib, a finding that is consistent with previous studies involving panobinostat-containing regimens7,14. This observation is likely due to the unique epigenetic mechanism of action of panobinostat, which targets multiple pathways that contribute to high-risk biology in MM and abrogates resistance to more established agents caused by epigenetic changes9,10,20.
AEs commonly associated with regimens incorporating panobinostat, bortezomib, and/or lenalidomide include neutropenia, diarrhea, PN, and thrombocytopenia2,13,21. The most commonly reported TRAEs in this study included diarrhea and PN, which were all G1/2. This finding contrasts with the phase 3 PANORAMA 1 study in which 25% of patients experienced G3/4 diarrhea and 18% experienced G3/4 PN5,6. The toxicity profile differences observed between the present study and PANORAMA 1 may be related to the different method of bortezomib administration (subcutaneous vs. intravenous) and the reduced dose of panobinostat (10–15 mg vs. 20 mg). At the MTD for pano-RVd, G3/4 AEs were experienced by 80% of patients, the majority of which were hematological. This high percentage of G3/4 AEs reflects chemotherapy-related bone marrow suppression in the context of a four-drug treatment regimen administered to patients who had received a median of four prior lines of therapy22. Importantly, no patients were withdrawn because of TRAEs, and 40% of patients were able to receive maintenance therapy as part of the study, including one patient who had received 74 cycles at the time of data cut-off. Collectively, these data demonstrate that a reduced panobinostat dose (10 mg) can be effective and tolerated as part of a quadruplet regimen.
The reported patient response to pano-RVd is similar to outcomes reported with pano-Rd in the phase 2 study by Chari et al. (ORR 44% vs. 41%)7. However, the inclusion of bortezomib within the pano-RVd regimen resulted, as expected, in a higher rate of AEs, particularly neuropathy and thrombocytopenia. The two study populations were similar in terms of exposure to prior therapies, and percentage of patients refractory to bortezomib and lenalidomide at time of study entry. However, the studies had different approaches to dosing panobinostat7. In the pano-Rd study, panobinostat was dosed every other week, rather than the 2-weeks-on/1-week-off schedule in the current study. This alternative dosing schedule used by Chari et al. may have enabled patients to tolerate higher doses of study treatment.
Anticipation of overlapping toxicities, particularly bone marrow suppression, led to the dosing strategy in this study, in which both lenalidomide and bortezomib were dose reduced relative to standard dosing of these agents. In spite of this strategy, dose reduction of lenalidomide, bortezomib and dexamethasone was required in 15%, 30%, and 30% of patients, respectively, and at least one treatment delay was required in 90% of patients. However, these reductions in dose enabled longer-term administration of all four agents, which presumably contributed to the sustained responses observed in some patients.
The small sample size is an important limitation of this study and inherent to phase 1 studies in general. While patients had received a substantial number of prior therapies, they were also relatively young, and thus it will be important to ascertain whether this quadruplet regimen could be tolerated in elderly patients23. However, the similar rates of TRAEs observed in patients <65 and ≥65 years old suggest that, indeed, pano-RVd may be tolerated in fit elderly patients.
This study was not designed or powered to definitively evaluate efficacy. However, the exploratory data reported herein are promising. Of note, in a previous study, pano-RVd was shown to have a favorable safety and efficacy profile in the front-line setting24. Panobinostat has also been shown to be effective and well tolerated when administered 1-week-on/1-week-off in a 28-day cycle in combination with lenalidomide and dexamethasone7 or with carfilzomib25, and once-weekly administration of bortezomib has been shown to improve the safety profile while maintaining efficacy26,27,28. As such, it would be of interest to evaluate a modified dosing regimen of pano-RVd using a 28-day cycle in which panobinostat is administered 1-week-on/1-week-off, along with weekly bortezomib, as such an approach may reduce the rates of thrombocytopenia and PN, while maintaining efficacy, and so translate efficiently into real-world practice23.
The relative lack of significant patient exposure to more recently approved agents such as carfilzomib, daratumumab, and elotuzumab is also a potential limitation of the study. Nonetheless, the results highlight the ability of panobinostat to re-sensitize patients to agents which, in most instances, they had previously become refractory to and were resistant. These data underscore the utility of panobinostat as an oral therapy with a unique, multifaceted mechanism of action that can partner with other agents to overcome resistance and enhance treatment response. Given the use of continuous/treat-to-progression therapy as a standard of care in MM, patients are more likely to become refractory to multiple agents after fewer lines of therapy, pointing to the need for additional treatment options for RRMM. As the dose and schedule of panobinostat are optimized, regimens incorporating this agent, such as pano-RVd, may contribute to further improvements in patient outcomes by targeting patterns of resistance to IMiDs and/or PIs, as well as to other novel agents29.
Data sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymized data may be granted following review.
References
Rajkumar, S. V. Multiple myeloma: 2018 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 93, 981–1114 (2018).
Durie, B. G. M. et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet 389, 519–527 (2017).
Pineda-Roman, M. et al. VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia 22, 1419–1427 (2008).
Siegel, D. S. et al. Improvement in overall survival with carfilzomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma. J. Clin. Oncol. 36, 728–734 (2018).
San-Miguel, J. F. et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 15, 1195–1206 (2014).
San-Miguel, J. F. et al. Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): a randomised, placebo-controlled, phase 3 trial. Lancet Haematol. 3, e506–e515 (2016).
Chari, A. et al. A phase 2 study of panobinostat with lenalidomide and weekly dexamethasone in myeloma. Blood Adv. 1, 1575–1583 (2017).
Mithraprabhu, S., Kalff, A., Chow, A., Khong, T. & Spencer, A. Dysregulated Class I histone deacetylases are indicators of poor prognosis in multiple myeloma. Epigenetics 9, 1511–1520 (2014).
Mitsiades, N. et al. Molecular sequelae of histone deacetylase inhibition in human malignant B cells. Blood 101, 4055–4062 (2003).
Mitsiades, C. S. et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc. Natl Acad. Sci. USA 101, 540–545 (2004).
Catley, L. et al. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood 108, 3441–3449 (2006).
FDA. FARYDAK prescribing information. (2015).
Laubach, J. P., Moreau, P., San-Miguel, J. F. & Richardson, P. G. Panobinostat for the treatment of multiple myeloma. Clin. Cancer Res. 21, 4767–4773 (2015).
Richardson, P. G. et al. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood 122, 2331–2337 (2013).
Laubach, J. et al. Phase 1b study of panobinostat in combination with lenalidomide, bortezomib, and dexamethasone in relapsed refractory multiple myeloma. J. Clin. Oncol. 34, 8014 (2016).
Laubach, J. P. et al. Efficacy and safety of oral panobinostat plus subcutaneous bortezomib and oral dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma (PANORAMA 3): an open-label, randomised, phase 2 study. Lancet Oncol. 22, 142–154. https://doi.org/10.1016/S1470-2045(20)30680-X (2021).
Tang, S. et al. Crucial role of HO-1/IRF4-dependent apoptosis induced by panobinostat and lenalidomide in multiple myeloma. Exp. Cell Res. 363, 196–207 (2018).
Rajkumar, S. V. et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 117, 4691–4695 (2011).
National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. (2009).
Issa, M. E. et al. Epigenetic strategies to reverse drug resistance in heterogeneous multiple myeloma. Clin. Epigenet. 9, 17 (2017).
Palumbo, A., Mateos, M. V., Bringhen, S. & San Miguel, J. F. Practical management of adverse events in multiple myeloma: can therapy be attenuated in older patients?. Blood Rev. 25, 181–191 (2011).
Moreau, P. et al. Adverse event management in patients with relapsed and refractory multiple myeloma taking pomalidomide plus low-dose dexamethasone: a pooled analysis. Eur. J. Haematol. 99, 199–206 (2017).
Richardson, P. G. et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 8, 109 (2018).
Manasanch, E. E. et al. Bortezomib, lenalidomide, and dexamethasone with panobinostat for front-line treatment of patients with multiple myeloma who are eligible for transplantation: a phase 1 trial. Lancet Haematol. 5, e628–e640 (2018).
Berdeja, J. G. et al. Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Haematologica 100, 670–676 (2015).
Bringhen, S. et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood 116, 4745–4753 (2010).
Reeder, C. B. et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood 115, 3416–3417 (2010).
Yagi H. F. A., et al. Once-weekly bortezomib in combination with reduced-dose dexamethasone and continuous low-dose oral cyclophosphamide for elderly patients with multiple myeloma. J. Hematol. Thrombo. Dis. 5, (2017).
Chim, C. S. et al. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia 32, 252–262 (2018).
Acknowledgements
The authors thank all patients involved in the study, as well as investigators and research staff in participating institutions. The authors thank Cara Valvona, PhD, of Watermeadow Medical, part of Ashfield Healthcare, Witney, UK, for providing medical writing support, funded by Secura Bio, Inc. San Diego, US, in accordance with Good Publication Practice guidelines. Funding: Novartis Pharmaceuticals Corporation funded this study. Secura Bio, Inc. funded the publication of this manuscript, including medical writing support.
Author information
Authors and Affiliations
Contributions
All authors were involved in data collection, analysis, and interpretation. All authors critically revised and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
J.P.L., J.M.R., K.C., K.M., D.W., and R.A.R. report no conflicts of interest. S.A.T. reports grants and personal fees from Celgene, grants and personal fees from Karyopharm, grants and personal fees from Sanofi, personal fees from Caelum, grants from Amgen, and grants from Janssen, outside the submitted work. C.S.M. discloses employment of a relative with Takeda; consultant/honoraria from Fate Therapeutics, Ionis Pharmaceuticals; past research funding from Novartis; as well as research funding outside the scope of this submitted work from Janssen/Johnson & Johnson, TEVA, EMD Serono, AbbVie, Karyopharm, Sanofi, and Arch Oncology. D.G. is a consultant to Secura Bio, Inc. P.G.R. reports grants from BMS, grants and honoraria (advisory committee member) from Oncopeptides, Celgene, Takeda, and Karyopharm; and honoraria (advisory committee member) from Janssen, Sanofi, and Secura Bio, Inc. outside the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was presented in part at the American Society of Clinical Oncology Annual Meeting, Chicago, June 2016, and the European Hematology Association Virtual Congress, June 2020.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Laubach, J.P., Tuchman, S.A., Rosenblatt, J.M. et al. Phase 1 open-label study of panobinostat, lenalidomide, bortezomib + dexamethasone in relapsed and relapsed/refractory multiple myeloma. Blood Cancer J. 11, 20 (2021). https://doi.org/10.1038/s41408-021-00407-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-021-00407-5