Abstract
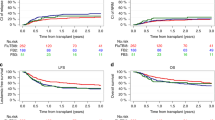
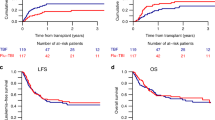
The optimal conditioning for patients with acute myeloid leukemia in first complete remission treated with allogeneic hematopoietic cell transplantation (allo-HCT) has not been defined so far. In this retrospective study, we compared two “reduced-toxicity” regimens: intravenous busulfan at a total dose of 9.6 mg/kg (3 days) + fludarabine (Bu3/Flu) and total body irradiation at a dose of 8 Gy + fludarabine (TBI8Gy/Flu). In the entire study cohort (n = 518), the probabilities of overall survival (OS), leukemia-free survival (LFS), relapse and non-relapse mortality (NRM) at 2 years for Bu3/Flu and TBI8Gy/Flu were 62% vs. 72.5% (p = 0.051), 59.5% vs. 65% (p = 0.15), 30% vs. 20% (p = 0.01), and 10% vs. 14% (p = 0.18), respectively. In multivariate model for patients <50 years old, TBI8Gy/Flu was associated with improved LFS (hazard ratio (HR) = 0.5, p = 0.04), OS (HR = 0.31, p = 0.004), and survival free from both graft-versus-host disease and relapse (HR = 0.55, p = 0.03), as well as tendency to reduced risk of relapse (HR = 0.53, p = 0.08). Among patients aged 50 years or older the use of TBI8Gy/Flu was associated with increased incidence of NRM (HR = 3.9, p = 0.0009), with no significant impact on other outcome measures. We conclude that the use of TBI8Gy/Flu as “reduced-toxicity” regimen may be advised in younger patients with AML referred for allo-HCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9.
Blaise D, Maraninchi D, Archimbaud E, Reiffers J, Devergie A, Jouet JP, et al. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-Cytoxan versus Cytoxan-total body irradiation as preparative regimen: a report from the Group d’Etudes de la Greffe de Moelle Osseuse. Blood. 1992;79:2578–82.
Ringdén O, Ruutu T, Remberger M, Nikoskelainen J, Volin L, Vindeløv L, et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood. 1994;83:2723–30.
Nagler A, Rocha V, Labopin M, Unal A, Ben Othman T, Campos A, et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen–a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31:3549–56.
Nagler A, Savani BN, Labopin M, Polge E, Passweg J, Finke J, et al. Outcomes after use of two standard ablative regimens in patients with refractory acute myeloid leukaemia: a retrospective, multicentre, registry analysis. Lancet Haematol. 2015;2:e384–392.
Copelan EA, Hamilton BK, Avalos B, Ahn KW, Bolwell BJ, Zhu X, et al. Better leukemia-free and overall survival in aml in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122:3863–70.
Passweg JR, Labopin M, Cornelissen J, Volin L, Socié G, Huynh A, et al. Acute Leukemia Working Party of the European Blood and Marrow Transplant Group (EBMT). Conditioning intensity in middle-aged patients with AML in first CR: no advantage for myeloablative regimens irrespective of the risk group-an observational analysis by the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2015;50:1063–8.
Hourigan CS, Dillon LW, Gui G, Logan BR, Fei M, Ghannam J et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol. 2020;38:1273–83.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transplant. 2020;55:1114–25.
Kanate AS, Nagler A, Savani B. Summary of scientific and statistical methods, study endpoints and definitions for observational and registry-based studies in hematopoietic cell transplantation. Clin Hematol Int. 2020;2:2–4.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
Fine JP, Gray RJ. A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Liu DH, Xu LP, Zhang XH, Wang Y, Yan CH, Wang JZ, et al. Substitution of cyclophosphamide in the modified Bucy regimen with fludarabine Is associated with increased incidence of severe pneumonia: a prospective, randomized study. Int J Hematol. 2013;98:708–15.
Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie D, et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. J Hematol Oncol. 2013;6:15.
Lee JH, Joo YD, Kim H, Ryoo HM, Kim MK, Lee GW, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013;31:701–9.
Rambaldi A, Grassi A, Masciulli A, Boschini C, Micò MC, Busca A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2015;16:1525–36.
Ben-Barouch S, Cohen O, Vidal L, Avivi I, Ram R. Busulfan fludarabine vs busulfan cyclophosphamide as a preparative regimen before allogeneic hematopoietic cell transplantation: systematic review and meta-analysis. Bone Marrow Transplant. 2016;51:232–40.
Mohty M, Malard F, Blaise D, Milpied N, Furst S, Tabrizi R, et al. Reduced-toxicity conditioning with fludarabine, once-daily intravenous busulfan, and antithymocyte globulins prior to allogeneic stem cell transplantation: results of a multicenter prospective phase 2 trial. Cancer. 2015;121:562–9.
Bornhäuser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–44.
Fasslrinner F, Schetelig J, Burchert A, Kramer M, Trenschel R, Hegenbart U, et al. Long-term efficacy of reduced-intensity versus myeloablative conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: retrospective follow-up of an open-label, randomised phase 3 trial. Lancet Haematol. 2018;5:e161–e169.
Socié G, Clift RA, Blaise D, Devergie A, Ringden O, Martin PJ, et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. Blood. 2001;98:3569–74.
Bredeson C, LeRademacher J, Kato K, Dipersio JF, Agura E, Devine SM, et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood. 2013;122:3871–8.
Buchali A, Feyer P, Groll J, Massenkeil G, Arnold R, Budach V. Immediate toxicity during fractionated total body irradiation as conditioning for bone marrow transplantation. Radiother Oncol. 2000;54:157–62.
Leiper AD. Late effects of total body irradiation. Arch Dis Child. 1995;72:382–5.
Potdar RR, Gupta S, Giebel S, Savani BN, Varadi G, Nagler A, et al. Current Status and perspectives of irradiation-based conditioning regimens for patients with acute leukemia undergoing hematopoietic stem cell transplantation. Clin Hematol Int. 2019;1:19–27.
Giebel S, Miszczyk L, Slosarek K, Moukhtari L, Ciceri F, Esteve J, et al. Extreme heterogeneity of myeloablative total body irradiation techniques in clinical practice: a survey of the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer. 2014;120:2760–5.
Andersson BS, Valdez BC. Pretransplant conditioning with fludarabine and IV busulfan, reduced toxicity and increased safety without compromising antitumor efficacy and overall treatment effect? Bone Marrow Transplant. 2016;51:919–20.
Andersson BS, Thall PF, Valdez BC, Milton DR, Al-Atrash G, Chen J, et al. Fludarabine with pharmacokinetically guided IV busulfan is superior to fixed-dose delivery in pretransplant conditioning of AML/MDS patients. Bone Marrow Transplant. 2017;52:580–7.
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Hoogard P. Frailty model for survival data. Lifetime Data Anal. 1995;1:255–73.
Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–1500.
Acknowledgements
We sincerely thank the centers of the EBMT for contributing patient information and data collection. Principal investigators of the contributing institutions are listed below.
Principle investigators of the contributing institutions
Sonja Martin15, Patrice Chevallier16, Andreas Neubauer17, Gandhi Damaj18, Yener Koc19, Arnold Ganser20, Matthew Collin21, Ibrahim Yakoub-Agha22, Hakan Ozdogu23, Mercedes Colorado Araujo24, Maija Itäla-Remes25, Kim Orchard26, Cecilia Isaksson27, Wolfgang Bethge28, Hans Martin29, Mahmoud Aljurf30, Edgar Faber31, Dolores Caballero32, Pavel Zák33, Xavier Leleu34, Jacques-Olivier Bay35, Pierre-Simon Rohrlich36, Nicolaus Kröger37, Anne Huynh38, Kerstin Schäfer-Eckart39, Noel Milpied40, Stig Lenhoff41, Aloysius Ho42, Jose Luis Bello López43, Nicola Mordini44, Bruno Lioure45, Kazimierz Halaburda46, Attilio Olivieri47, Tobias Gedde-Dahl48, Rachel Protheroe49, Johanna Tischer50, Matthias Klammer51, Johannes Clausen52, Victoria Potter53, Marco Ladetto54, Herve Tilly55, Eric Deconinck56, Arne Brecht57, Lutz Peter Müller58, Thomas Heinicke59, Juan Pio Torres Carrete60, Ali Bazarbachi61, Péter Reményi62, Marie Thérèse Rubio63, Renato Fanin64, Jose Antonio Pérez-Simón65, Murawski Niels66, J. L. Diez-Martin67, Mutlu Arat68, Olivier Hermine69, Gerard Socié70, Jan J. Cornelissen71, Stella Santarone72, Denis Guyotat73, Claude Eric Bulabois74, Paolo Bernasconi75, Jan-Erik Johansson76, Radovan Vrhovac77, Hildegard Greinix78, José Luis López Lorenzo79, Shashikant Apte80, Charles Craddock81, Guido Kobbe82, Mohsen Al Zahrani83, Peter Dreger84, Andrzej Lange85, Abdelghani Tbakhi86, Ellen Meijer87, Carlos Vallejo Llamas88, Josep Maria Ribera Santasusana89, Paolo Corradini90, Fabio Benedetti91, Alessandro Rambaldi92, Virginie Gandemer93, Jean-Valère Malfuson94, Ain Kaare95, Antonio Risitano96, Mario Petrini97, Carmine Selleri98, Depei Wu99.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the contributing institutions are listed below Acknowledgements.
Rights and permissions
About this article
Cite this article
Giebel, S., Labopin, M., Sobczyk-Kruszelnicka, M. et al. Total body irradiation + fludarabine compared to busulfan + fludarabine as “reduced-toxicity conditioning” for patients with acute myeloid leukemia treated with allogeneic hematopoietic cell transplantation in first complete remission: a study by the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant 56, 481–491 (2021). https://doi.org/10.1038/s41409-020-01050-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01050-7
This article is cited by
-
Reduced toxicity (FluBu3) versus myeloablative (BuCy) conditioning in acute myeloid leukemia patients who received first allogeneic hematopoietic stem cell transplantation in measurable residual disease-negative CR1
Bone Marrow Transplantation (2024)
-
Myeloablative conditioning regimens in adult patients with acute myeloid leukemia undergoing allogeneic hematopoietic stem cell transplantation in complete remission: a systematic review and network meta-analysis
Bone Marrow Transplantation (2023)
-
Total body irradiation plus fludarabine versus busulfan plus fludarabine as a myeloablative conditioning for adults with acute myeloid leukemia treated with allogeneic hematopoietic cell transplantation. A study on behalf of the Acute Leukemia Working Party of the EBMT
Bone Marrow Transplantation (2023)
-
Total body irradiation versus busulfan based intermediate intensity conditioning for stem cell transplantation in ALL patients >45 years—a registry-based study by the Acute Leukemia Working Party of the EBMT
Bone Marrow Transplantation (2023)
-
Strahlentherapeutische Behandlung von Leukämien
best practice onkologie (2022)