Abstract
Introduction
Cisplatin and gemcitabine (CisGem) are standard chemotherapy for advanced biliary tract cancer (BTC). The MEK inhibitor selumetinib showed synergy with gemcitabine when administered sequentially in BTC. This randomised Phase 2 trial aimed to assess the efficacy of sequential or continuous selumetinib with CisGem.
Methods
Patients with advanced BTC received CisGem; arm A included selumetinib every day, arm B: selumetinib, days 1–5, 8–19 each cycle. Arm C received CisGem alone. Selumetinib was dosed at 75 mg BID but amended to 50 mg BID due to toxicity.
Results
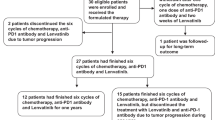
In all, 51 participants were evaluable for response. No significant difference was seen in mean change in tumour size at 10 weeks between arms A and C (−7.8% vs −12.8%, P = 0.54) or arms B and C (−15% vs −12.8%, P = 0.78). There was no difference in median progression-free survival (6.0, 7.0, 6.3 months, P > 0.95) or overall survival (11.7, 11.7, 12.8 months, P = 0.70) for arms A, B and C, respectively. More participants experienced grade 3–4 toxicities in selumetinib-containing arms. More participants in arm A required chemotherapy dose reductions (P = 0.01) with lower chemotherapy dose intensity during the first 10 weeks.
Conclusion
Adding sequential or continuous selumetinib to CisGem failed to improve efficacy and increased toxicity in patients with advanced BTC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Supporting data for this study are held by the Drug Development Programme, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
References
Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–7. https://doi.org/10.1053/jhep.2001.25087.
Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pr Res Clin Gastroenterol. 2015;29:221–32. https://doi.org/10.1016/j.bpg.2015.02.003.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl J Med. 2010;362:1273–81. https://doi.org/10.1056/NEJMoa0908721.
Wardell CP, Fujita M, Yamada T, Simbolo M, Fassan M, Karlic R, et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol. 2018;68:959–69. https://doi.org/10.1016/j.jhep.2018.01.009.
Haber PK, Sia D. Translating cancer genomics for precision oncology in biliary tract cancers. Discov Med. 2019;28:255–65.
Tannapfel A, Sommerer F, Benicke M, Katalinic A, Uhlmann D, Witzigmann H, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706–12. https://doi.org/10.1136/gut.52.5.706.
Tannapfel A, Benicke M, Katalinic A, Uhlmann D, Köckerling F, Hauss J, et al. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721–7. https://doi.org/10.1136/gut.47.5.721.
Bridgewater J, Lopes A, Beare S, Duggan M, Lee D, Ricamara M, et al. A phase 1b study of Selumetinib in combination with Cisplatin and Gemcitabine in advanced or metastatic biliary tract cancer: the ABC-04 study. BMC Cancer. 2016;16:153 https://doi.org/10.1186/s12885-016-2174-8.
Bekaii-Saab T, Phelps MA, Li X, Saji M, Goff L, Kauh J, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol J Am Soc Clin Oncol. 2011;29:2357–63. https://doi.org/10.1200/JCO.2010.33.9473.
Xu J, Knox JJ, Ibrahimov E, Chen E, Serra S, Tsao M, et al. Sequence dependence of MEK inhibitor AZD6244 combined with gemcitabine for the treatment of biliary cancer. Clin Cancer Res. 2013;19:118 LP–127. https://doi.org/10.1158/1078-0432.CCR-12-2557.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Karrison TG, Maitland ML, Stadler WM, Ratain MJ. Design of Phase II cancer trials using a continuous endpoint of change in tumor size: application to a study of sorafenib and erlotinib in non–small-cell lung cancer. JNCI J Natl Cancer Inst. 2007;99:1455–61. https://doi.org/10.1093/jnci/djm158.
Lowery MA, Bradley M, Chou JF, Capanu M, Gerst S, Harding JJ, et al. Binimetinib plus gemcitabine and cisplatin phase I/II trial in patients with advanced biliary cancers. Clin Cancer Res. 2019;25:937–45. https://doi.org/10.1158/1078-0432.CCR-18-1927.
Greystoke A, Steele N, Arkenau H-T, Blackhall F, Haris NM, Lindsay CR, et al. SELECT-3: a phase I study of selumetinib in combination with platinum-doublet chemotherapy for advanced NSCLC in the first-line setting. Br J Cancer. 2017;117:938–46. https://doi.org/10.1038/bjc.2017.271.
Moehler M, Maderer A, Schimanski C, Kanzler S, Denzer U, Kolligs FT, et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: a double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur J Cancer. 2014;50:3125–35. https://doi.org/10.1016/j.ejca.2014.09.013.
Valle JW, Wasan H, Lopes A, Backen AC, Palmer DH, Morris K, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol. 2015;16:967–78. https://doi.org/10.1016/S1470-2045(15)00139-4.
Demols A, Borbath I, Van den Eynde M, Houbiers G, Peeters M, Marechal R, et al. Regorafenib after failure of gemcitabine and platinum-based chemotherapy for locally advanced/metastatic biliary tumors: REACHIN, a randomized, double-blind, phase II trial. Ann Oncol. 2020;31:1169–77. https://doi.org/10.1016/j.annonc.2020.05.018.
Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–84. https://doi.org/10.1016/S1470-2045(20)30109-1.
Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796–807. https://doi.org/10.1016/S1470-2045(20)30157-1.
Subbiah V, Lassen U, Élez E, Italiano A, Curigliano G, Javle M, et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21:1234–43. https://doi.org/10.1016/S1470-2045(20)30321-1.
Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;38:1–10. https://doi.org/10.1200/JCO.19.02105.
Funding
This study was funded as externally sponsored research by AstraZeneca, who provided investigational selumetinib. Additional funding was provided by the Princess Margaret Cancer Foundation.
Author information
Authors and Affiliations
Contributions
MD—conceptualisation, methodology, investigation and manuscript preparation; VT—investigation, manuscript review; MM—conceptualisation, methodology and manuscript review; RJ—investigation and manuscript review; DH—conceptualisation, investigation and manuscript review; EC—investigation and manuscript review; ND—investigation and manuscript review; PT—investigation and manuscript review; HWS—investigation and manuscript review; GO’K—investigation and manuscript review; SD—resources and investigation; LW—formal analysis; TP—data curation and project administration; JK—conceptualisation, methodology, investigation and manuscript review.
Corresponding author
Ethics declarations
Competing interests
MD received research funding from AstraZeneca and Merck, and speaking honoraria from AstraZeneca, Roche, Merck, Boehringer Ingelheim, Eisai and Takeda. VT received research funding from AstraZeneca, Eisai, Exelixis, Ipsen, Merck, Roche, travel support from Amgen, and speaking honoraria from AstraZeneca, Eisai, Ipsen and Roche. MM received research support from Servier, Ipsen, and NuCana, travel/accommodation support from Bayer and Ipsen, speaker honoraria from Pfizer, Ipsen, NuCana, and Mylan, and consulted for Celgene, Ipsen, Sirtex, Baxalta and Incyte. RJ received research funding from AstraZeneca, Boston Biomedical, Bristol-Myers Squibb, Lilly, Merck and Novartis, and consulted for Ipsen and Novartis. EC received research funding from AstraZeneca, Boston Biomedical, Bristol-Myers Squibb, Merck and Novartis, and speaking honoraria from Bayer, Eisai and Taiho. ND received speaker honoraria from Celgene and Sanofi, and consulted for Celgene and Incyte. PT received travel support from Amgen and consulted for Amgen, AstraZeneca, Celgene, Eisai, Genomic Health International, Merck, Pfizer, Taiho and Teva. HWS received research funding from Abbvie and Bristol-Meiers Squibb. GO’K received speaking honoraria from Eisai and Roche, travel support from AstraZeneca, and consulted for Eisai and Roche. JK received research funding from and consulted for AstraZeneca, Ipsen and Merck, and speaking honoraria from Novartis and Roche. The remaining authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Research Ethics Boards of the Princess Margaret Cancer Centre and Tom Baker Cancer Centre. All patients provided informed consent and the study was carried out in accordance with the Declaration of Helsinki. The trial was registered with clinicaltrials.gov, ID NCT02151084.
Consent to publish
None.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doherty, M.K., Tam, V.C., McNamara, M.G. et al. Randomised, Phase II study of selumetinib, an oral inhibitor of MEK, in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer. Br J Cancer 127, 1473–1478 (2022). https://doi.org/10.1038/s41416-022-01903-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01903-6