Abstract
Introduction
CONTACT is a national multidisciplinary study assessing the impact of the COVID-19 pandemic upon diagnostic and treatment pathways among patients with pancreatic ductal adenocarcinoma (PDAC).
Methods
The treatment of consecutive patients with newly diagnosed PDAC from a pre-COVID-19 pandemic cohort (07/01/2019-03/03/2019) were compared to a cohort diagnosed during the first wave of the UK pandemic (‘COVID’ cohort, 16/03/2020-10/05/2020), with 12-month follow-up.
Results
Among 984 patients (pre-COVID: n = 483, COVID: n = 501), the COVID cohort was less likely to receive staging investigations other than CT scanning (29.5% vs. 37.2%, p = 0.010). Among patients treated with curative intent, there was a reduction in the proportion of patients recommended surgery (54.5% vs. 76.6%, p = 0.001) and increase in the proportion recommended upfront chemotherapy (45.5% vs. 23.4%, p = 0.002). Among patients on a non-curative pathway, fewer patients were recommended (47.4% vs. 57.3%, p = 0.004) or received palliative anti-cancer therapy (20.5% vs. 26.5%, p = 0.045). Ultimately, fewer patients in the COVID cohort underwent surgical resection (6.4% vs. 9.3%, p = 0.036), whilst more patients received no anti-cancer treatment (69.3% vs. 59.2% p = 0.009). Despite these differences, there was no difference in median overall survival between the COVID and pre-COVID cohorts, (3.5 (IQR 2.8–4.1) vs. 4.4 (IQR 3.6–5.2) months, p = 0.093).
Conclusion
Pathways for patients with PDAC were significantly disrupted during the first wave of the COVID-19 pandemic, with fewer patients receiving standard treatments. However, no significant impact on survival was discerned.
Similar content being viewed by others
Introduction
The COVID-19 pandemic has had an unprecedented impact on healthcare systems, with major impact upon delivery of non-COVID-related services [1]. Pressure on healthcare services to prioritise care for those with COVID-19 infection inevitably led to a reduction in service availability for patients with other conditions [2]: an estimated 28 million operations were cancelled worldwide in the first 12 weeks of the pandemic, for example [3].
Cancer patients were considered particularly vulnerable to COVID-19, due to increased risk of infection and mortality [4,5,6,7]. Initial data suggested that infection with COVID-19 in the perioperative period, or when receiving anti-cancer drug treatment, was associated with high rates of mortality [8, 9]. Consequently, at the start of the UK COVID-19 pandemic, guidelines were generated by both national and international groups regarding changes to standard cancer patient management [10, 11]. Specifically for patients with PDAC, the European Society for Medical Oncology (ESMO) [12] and UK Consensus Statement for treatment of pancreatic cancer [9] guidance made recommendations on modifying patient pathways, generally anticipating less or deferred surgery, a more cautious approach to the use of systemic therapy particularly in the case of unresectable disease, and an opportunity to explore hitherto non-standard hypofractionated radiotherapy regimens. Subsequent data did not confirm anticancer drug treatment to be associated with increased mortality, hence oncologists revised the initial plans to de-escalate use of these therapies in the second half of 2020 and subsequent waves of the pandemic [13].
PDAC is associated with some of the worst outcomes from any form of cancer [14, 15]. The benefits of anti-cancer interventions are modest relative to those achieved for most other common cancers, and in an unprecendented situation when healthcare resources needed prioritising towards those most likely to benefit (both with regard to COVID-19 infection and to cancer), there was a risk that patients with PDAC might have been particularly vulnerable to changes in standard of care that might in fact worsen their disease outcomes. The aim of the CONTACT study was to compare the recommended and received treatments among patients diagnosed with PDAC during the first wave of the COVID-19 pandemic with a similar patient cohort diagnosed in early 2019, pre-pandemic. The primary aim was to determine whether diagnosis of PDAC during the pandemic was associated with a reduction in standard treatment of PDAC, secondary aims were to compare treatment intent to received treatment and survival at one year.
Methods and analysis
The CONTACT study is reported according to Strengthen the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [16].
The primary objective was to compare treatment(s) received by patients with PDAC diagnosed during the first wave of the COVID-19 pandemic in the UK, compared with a similar cohort diagnosed prior to the pandemic. Secondary objectives include assessment of the diagnostic pathway, recommended treatment, times to treatment and 12-month outcomes, compared to a pre-pandemic cohort.
Setting and study design
This was a national, observational cohort study that implemented a collaborative research model with data collection undertaken by trainee doctors. A novel, mixed prospective and retrospective design, with retrospective case identification of both cohorts was used. The pre-COVID cohort comprised patients diagnosed with PDAC during an 8 week period, between 07 January to 03 March 2019. The COVID cohort comprised patients diagnosed with PDAC during an 8 week period, between 16 March to 10 May 2020. All patients were followed up for 12 months, so the data collection on the pre-pandemic cohort predated the start of the UK COVID-19 pandemic.
All UK hospitals (n = 156) with an established PDAC multidisciplinary team (MDT) were eligible to join the study and were invited through email invitation and by invitation through specialty organisations (Pancreatic Society of Great Britain and Ireland, Association of Upper Gastrointestinal Surgery, and Great Britain and Ireland Hepatopancreatobiliary Association). PDAC treatment in the UK is via a ‘hub-and-spoke’ network whereby each specialist surgical ‘hub’ is networked to its ‘spoked’ hospitals that do not provide surgery. The definition of ‘specialist’ centre henceforth, refers to a hospital in which pancreatic surgery is available. Across most networks chemotherapy is delivered at the local ‘spokes’, although in two centres, the delivery of chemotherapy has been largely centralised. Volunteer trainee regional leads were recruited to oversee data collection at hospitals linked to their network. Medical students worked with the study coordinators to support the regional leads, facilitate communication and ensure data was collected according to the study protocol.
All adult patients (≥18 years old) with suspected PDAC presenting during the case identification periods and discussed at pancreatic cancer MDTs were included in this study. In the two regional sites (Manchester and Liverpool) with both centralised surgery and chemotherapy services, all patients were identified at the regional site. For all other sites, data was entered by the site where the patient was both initially diagnosed with PDAC and subsequently received ongoing treatment, such as chemotherapy given at the local site, ensuring treatment throughout the patient pathway as well as follow-up data was captured.
Inclusion and exclusion criteria
Before analysis, the data was screened to ensure all included patients fulfilled the inclusion criteria for the study. Patients were included if they were over the age of 18 years, they had presented initially to the reporting hospital with suspected PDAC and had had an initial CT scan, indicative of such. Patients were excluded if subsequent investigations or treatment confirmed the diagnosis was not PDAC, or the data available was incomplete. A minimum data requirement of: receipt of index CT scan, MDT recommendation and treatment received, was used.
Variables and data collection
The following data was collected: (1) baseline demographics, (2) diagnostic and staging tests, (3) management (both recommended treatment at the MDT and actual treatment received), and (4) survival at 12 months. Data was collected from routine medical records and no patients were contacted. Anonymised patient data was uploaded to a REDCap database [17, 18].
Statistical methods
Descriptive statistics were used to display demographic variables. Continuous data was expressed as median (interquartile range; IQR), and categorical variables presented as numbers and/or percentages. Chi-squared test was used to test for significance in categorical variables whilst Mann–Whitney-U were used for ordinal and continuous data. Binary regression analysis was used to calculate odds ratios and corresponding 95% confidence intervals. Cox-regression analysis was used for hazard ratio calculation for 12-month survival, and a Kaplan–Meier logistic regression curve used to display the results. Due to data protection limitations, only the week of death was able to be collected. To mitigate bias, when calculating survival only, the date of the initial CT scan was adjusted to the Monday of that week, and ‘day of death’ assigned to the Monday of the week of death collected. A p-value of <0.05 was considered significant.
Ethics and dissemination
Patient consent was not required for this study, as only routinely collected datapoints were collected by members of the local healthcare team, and the centrally analysed data was anonymous. This was confirmed using the national UK decision-making tool of the NHS Health Research Authority and the Medical Research Council [19]. The CONTACT study was locally registered as a clinical audit or service evaluation project at each participating site prior to patient identification and data collection.
Results
Baseline demographics
After screening 1261 possible cases and applying exclusion criteria, 984 cases with PDAC treated across 96 hospitals were included in the final analysis (pre-COVID: n = 484 and COVID: n = 501). 22 hospitals were specialist pancreatic centres (184 patients vs. 200 patients), and two networks centralised delivery of anti-cancer therapy (31 patients vs. 31 patients). There were no significant differences in median age (73 years, range: 65–80 vs. 73 years, range: 66–81), gender (268, 53.4% vs. 258, 53.4% male), or performance status (PS: 320, 63.8% vs. 290, 59.9% PS 0–1) between the COVID and pre-COVID cohorts. The vast majority of patients were considered to be on a non-curative pathway (424, 84.6% of COVID vs. 389, 80.5% of pre-COVID cohort). A complete list of baseline characteristics is shown in Table 1.
Staging investigations and treatment of jaundice
All patients underwent at least one CT scan as per the inclusion criteria. Patients in the COVID cohort were less likely to undergo any further staging tests compared with pre-COVID (148/501, 29.5% vs. 180/486, 37.2%; p = 0.01). Specifically, there was a reduction in the use of EUS (OR: 0.65, 95%CI: 0.48–0.88; p = 0.006) and MRI (OR: 0.66, 95%CI: 0.44–0.99; p = 0.043) compared to those patients in the pre-COVID cohort, and fewer patients had a histologically confirmed diagnosis of malignancy (OR: 0.72, 95%CI: 0.56–0.93, p = 0.011). There was no difference in the time from diagnosis to the various staging investigations between the cohorts. There was no difference in the proportion of jaundiced patients, treatment of jaundice or time to treating jaundice between the cohorts (Table 2).
MDT-recommended treatment of PDAC
Across the whole study population (pre-COVID n = 483; COVID n = 501) more patients were recommended best supportive care (i.e., no surgery or non-surgical anticancer treatment) in the COVID cohort (44.5% vs. 34.4% p = 0.001; OR 1.53 95%CI 1.18–1.98), fewer were recommended cancer resection surgery (8.4% vs. 14.9% p = 0.002; OR 0.52 95%CI 0.35–0.78), while more were recommended upfront chemotherapy (UFC) (7.0% vs. 4.6% p = 0.105; OR 1.57, 95%CI 0.91–2.7) in the COVID cohort compared to the pre-COVID cohort. There was also a non-significant reduction in the recommendation to use palliative chemotherapy (40.1% vs. 46.2% p = 0.056; OR 0.78, 95%CI 0.61–1.01) in the COVID cohort.
Actual treatment of PDAC
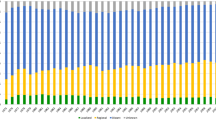
Across the whole study population, more patients received best supportive care (67.3% vs. 59.2% p = 0.009; OR 1.42, 95%CI: 1.09–1.84), fewer underwent cancer resection surgery (6.4% vs. 9.3% p = 0.036; OR 0.64, 95%CI: 0.37–0.97) and more received UFC (5.8% vs. 3.3% p = 0.063; OR 1.81) in the COVID cohort compared with the pre-COVID cohort. There was also a non-significant trend in reduction in the use of palliative chemotherapy (17.4% vs. 21.3% p = 0.116; OR 0.76, 95%CI: 0.56–1.07) in the COVID cohort. The intended and actual treatments received for the whole cohort are summarised in Fig. 1.
Survival analysis
There was no difference in survival between the two cohorts overall, with a median survival of 3.5 (IQR 2.8–4.1) months in the COVID cohort vs. 4.4 (IQR 3.6–5.2) months in the pre-COVID cohort (HR 1.132, 95%CI 0.980–1.037; p = 0.093) (Fig. 2); 23.4% and 26.5% of the COVID and pre-COVID cohorts were alive at 12 months. Comparing COVID and pre-COVID cohorts, 64.9% and 63.8% of patients within the potentially curative pathway were alive at 12-months (HR: 0.98, 95%CI: 0.59–1.62; p = 0.932) whilst 9.9% and 11.3% of patients within the non-curative pathway were alive at 12-months (HR: 1.055, 95%CI: 0.85–1.31; p = 0.624).
Within group treatment
Patients could follow either a pathway with curative or non-curative intent and these are now considered separately.
Curative intent pathway
Fewer patients on a curative pathway in the COVID cohort vs. pre-COVID cohort were recommended surgery (42/77, 54.5% vs. 72/94, 76.6% p = 0.001) whilst more were recommended UFC (35/77, 45.5% vs. 22/94, 23.4% p = 0.002). However, in the groups recommended surgery there was no difference in the proportion that were resected (22/42, 52.3% vs. 41/72, 56.9%: COVID vs. pre-COVID, respectively; p = 0.636). Thus, 47.7% patients in the COVID cohort and 43.1% patients in the pre-COVID cohort recommended surgery were not actually operated on.
Patients with good PS (0–1) were more likely to be recommended to receive UFC during the pandemic, rather than immediate surgery (46.5% vs. 20.7%, p < 0.001). The number of patients recommended UFC with resectable tumours (rather than borderline or locally advanced) also increased during the pandemic, though non-significantly (24.2% vs. 5.9%, p = 0.109).
There was no difference in the proportion of jaundiced patients or those proceeding direct to surgery with jaundice between the cohorts. There was no difference in the use of, or time to, adjuvant therapy, but patients were more likely to receive single agent chemotherapy in the COVID cohort (6/16, 38% vs. 3/29, 8%, p = 0.038). Of those recommended UFC, only 20% in the COVID (n = 7/35) and 18% in the pre-COVID (n = 4/22) cohorts were resected. A further 11% and 9% in the COVID (n = 4/35) and pre-COVID (n = 2/22) cohorts underwent attempted surgery but failed resection after UFC (Table 3). There was no difference in vascular resection rate, T or N status between the operated cohorts. There was no difference in the rates of local or metastatic recurrence, of the time to recurrence between the operated cohorts, nor 90-day-mortality after resection surgery (2/29, 6.9% vs. 1/45, 2.2%, p = 0.557).
Non-curative intent pathway
Fewer patients on a non-curative pathway in the COVID cohort were recommended palliative therapy (COVID 201/424, 47.4% vs. pre-COVID 223/389, 57.3% OR: 1.11 95%CI: 0.96–1.85; p = 0.001), fewer patients actually received any palliative therapy (20.5% vs. 26.5%; OR:0.72 95%CI: 0.52–0.99, p = 0.045), and fewer received palliative chemotherapy (18.9% vs. 24.7%; OR: 0.71 95%CI: 0.51–0.99, p = 0.044). In almost half of cases in both cohorts (48.6% COVID vs. 45.4% pre-COVID), the reason given for patients not receiving recommended palliative therapy was frailty. The next most common reasons were recurrence/progression (26.4% COVID vs. 29.0% pre-COVID) and patient choice (18.6% COVID vs. 20.2% pre-COVID). COVID-19 itself was only cited as a reason in 2.3% of the COVID cohort.
There was no difference in rates of palliative radiotherapy between the COVID and pre-COVID cohorts (3.5% vs. 3.3%; p = 0.878), or reasons provided why patients were deemed non-curative/unresectable, p = 0.363. Among patients receiving palliative chemotherapy, there was no difference in the first-line agents received, the proportion of patients completing all cycles of chemotherapy, median time to starting chemotherapy, or the proportion of patients receiving second line therapy. This data is described in detail in Table 4. Patients with poor performance status (≥2) were less likely to be recommended to receive palliative therapy during the pandemic (25.1% vs. 38.1%, p = 0.009). There was no difference for the reasons given why patients did not receive any therapy between the cohorts (COVID n = 337/424, 79.5%, pre-COVID n = 286/389, 73.5%; p = 0.277).
Discussion
The CONTACT study was an observational study of two cohorts of consecutive patients diagnosed with PDAC across UK hospitals with 12 months follow-up, before and during the first wave of the COVID-19 pandemic. Importantly, there was no overlap of the follow-up period for the 2019 cohort and the start of the pandemic. Both cohorts shared similar demographics typical of this disease, being relatively elderly (40% over the age of 75 years, 25% over the age of 80 years) and frail (35–40% PS 2–3) populations, and only 1 in 5 being considered potentially curable at the time of initial assessment.
The key CONTACT study findings confirmed that access to surgery during the pandemic was significantly curtailed and patients were offered up-front chemotherapy as a bridging treatment modality, aimed at deferring planned surgery, as recommended in the UK consensus recommendations [9]. In addition, the proportion of patients on a non-curative pathway who received palliative therapy was lower compared with the pre-COVID cohort. Despite these differences, survival at 12-months for the two cohorts overall did not differ significantly. Other notable observations are discussed in three sections: diagnosis, curative intent pathway and non-curative intent pathway.
The National Institute of Health and Care Excellence (NICE) guidance recommends resectional surgery rather than pre-operative drainage among jaundiced patients with potentially resectable disease [11]. However, ESMO guidelines for management of PDAC during COVID-19 recommended prompt resolution of jaundice to create better conditions for subsequent management, be that curative or palliative [12]. The proportion of jaundiced patients proceeding direct to surgery was low, and did not differ between the cohorts. Although there was no significant change in the apparent treatment of jaundice, which requires invasive and possibly aerosol generating procedures, there was a reduction in the use of additional staging tests (EUS and MRI) among patients in the COVID cohort, raising concern that some patients may have been inadequately managed.
Among patients treated with curative intent there was an increase in the use of UFC during COVID. Whilst NICE guidance predating COVID-19 recommends neoadjuvant chemotherapy (NAT) to be used within a trial-based setting, emerging evidence has demonstrated potential benefits of NAT in patients with potentially resectable disease [20]. NAT may increase the number of patients completing their treatment course [21]. During the pandemic, with increased risk of perioperative morbidity, and reducing in access to surgery [22], UFC was by necessity used as a ‘bridging’ modality to delay surgery and hence became a real-time strategy to ‘test cancer biology’ [23], beyond its use with pure neoadjuvant intent. However, immunosuppressive risks of chemotherapy must also be considered, particularly in this vulnerable cohort, with an increased risk of mortality with COVID-19 infection, when compared to a non-cancer population [24]. Regardless of the cohort, the rates of resectional surgery following pre-operative chemotherapy were very low, with 20% or fewer patients being resected ultimately, either during or pre-COVID. These UK data compare poorly with the 61% resection rate reported in the Dutch PREOPANC phase III trial for patients with resectable or borderline resectable disease receiving neoadjuvant chemoradiotherapy [25]. The reasons for discrepancy in these figures are not entirely clear, but most likely reflect real-world data, lacking the rigors of a prospective controlled trial. UFC may well have been recommended for patients with more extensive, locally advanced disease, in whom resection rates are known be much lower.
The rapid uptake of UFC, as well as hypofractionated radiotherapy, not previously commissioned has demonstrated the potential value of real-world evaluation of new patient pathways that would historically have only be tested within prospective randomised trials. Clinical trials are expensive, time consuming and, as demonstrated by the ESPAC5F randomised trial of NAT, can be extremely challenging to recruit to [26]. It is noteworthy that since the pandemic, NAT is being adopted for patients with borderline resectable PDAC and outcome data for this patient group should be formally assessed.
For those patients completing surgery, whilst there was no difference in the proportion of patients receiveing adjuvant chemotherapy, there was a difference in the regimens used, with an increase in the use of single agent chemotherapy regimens in the COVID cohort. The NICE PDAC management guidelines predate the PRODIGE-24 trial demonstrating superiority of adjuvant mFOLFIRINOX compared with adjuvant gemcitabine, but do recommend combination chemotherapy (gemcitabine+capecitabine) in preference to gemcitabine, based on randomised trial evidence of benefit [11]. The decision to offer patients single agent rather than combination chemotherapy may well have been a deliberate decision to reduce risk of myelosuppression and safeguard patients. Reassuringly, the proportion of patients starting and completing adjuvant therapy was high and did not differ between the cohorts. The number of patients commencing adjuvant therapy in the present study, compares favourably to other national-scale data [21, 27,28,29,30]. Finally, there was no difference in time to initiation of adjuvant therapy, with median weeks to therapy in both cohorts being within 12-weeks of surgery, benefits of which are evidenced by data from published ESPAC trials [31, 32].
For patients on a non-curative pathway, there was a significant reduction in the use of palliative chemotherapy during the pandemic compared to pre-pandemic use. FOLFIRINOX was used first-line in the majority of cases in both cohorts, as per NICE guidance [11], and amongst patients who received palliative chemotherapy, there was a significant increase in FOLFIRINOX use (p = 0.044). Given that the number of patients recommended surgery was lower in the COVID cohort, whilst significantly more patients were recommended UFC, it is likely that more good performance status patients with locally advanced PDAC were being recommended and subsequently offered multi-agent chemotherapy. The lower use of palliative chemotherapy during the COVID-19 first wave may be explained by the perceived greater risk of harm from COVID-19 infection due to immunosuppression whilst on chemotherapy. A national consensus recommended a highly selective and individualised approach to palliative chemotherapy in the pandemic, with early response assessments encouraged to limit risk [9]. Interestingly the UK guidance was also to limit offering 2nd line chemotherapy, but in both patient cohorts the proportion of patients receiving 2nd line therapy was the same, at 12.5%. Similar rates of cycle completion before and during the pandemic may indicate persistent, careful selection of patients who would both benefit from, and tolerate palliative chemotherapy.
The proportion of patients receiving the intervention they were initially recommended at MDT was low both before and during the pandemic, particularly for those recommended to surgery (COVID 59.5% vs. pre-COVID 73.6%) and palliative therapy (COVID 43.3% vs. pre-COVID 46.2%). These data demonstrate limitations of MDT assessments, especially in the palliative setting, when key information regarding factors such as patient frailty and co-morbidities are generally lacking, but play an important part in determining fitness for intervention in an elderly population. Furthermore, the aggressive nature of PDAC can rapidly change patients’ overall health status and influence the intended treatment recommendations.
Whilst the pandemic forced changes upon PDAC patient treatment pathways, which otherwise would not have occurred over the past two years, some are not entirely detrimental; presenting opportunities to evaluate novel or emerging interventions. Some changes in managing patients have been serendipitous as they may not have occurred otherwise, such as a move to more remote consultations on an individualised basis. Exploring the role and outcomes of UFC in the pre-operative setting is another example; however, as seen in this study, the rates of resection were low and thus more work is needed to evaluate the role of UFC, specifically NAT, in managing early stages of PDAC. NHS England has drafted new guidance for faster diagnosis of PDAC. Soon to be published, implementation of this guidance will be essential to improving patient pathways and remove the delays that likely impact on patients accessing optimal care.
Given the changes to PDAC patient management during COVID vs. pre-COVID, it is surprising, but somewhat reassuring, that overall survival was not affected. It is possible that this may be due to a type 2 error, as only the minority of patients (~35%) received any form of treatment and the cohorts were relatively small, being identified over a 2 month period. On the other hand, the absolute benefits of both surgical and non-surgical interventions for this aggressive cancer type must be acknowledged to be modest, and these data give impetus for the urgent need to pursue research into its biology, understanding the drivers and develop innovative approaches to treatment as well as prevention.
Regional variation was not assessed in this study, but there was evidence of variation in practice between specialist and non-specialist sites and between regions in the RICOCHET national prospective PDAC audit [33, 34]. Further work will be required to assess whether different models of care were more or less successful at maintaining treatment throughout the pandemic with consequent impact on patient outcomes.
Limitations and recommendations
It is a requirement to discuss all suspected and newly diagnosed cancer patients at MDT, therefore case ascertainment of PDAC patients is expected to be 100% at participating sites. Datapoints in this study were not validated, but it is known that when using the same network to capture data in the RICOCHET national PDAC audit that data accuracy exceeded 95% [33, 34]. Even so, the size of this study population is relatively modest. Not all centres with an MDT contributed data, so this is not a complete picture of care across the UK, but data is drawn from almost every specialist centre and from all the devolved nations, not just England. Given the relatively low rates of surgery among those affected by PDAC, the small size of some subgroups within this study limit interpretation. Access to national datasets might be expected to better quantify changes in pathways and treatments particularly on the key outcome of patient survival. The data are, however, strong enough to inform practice, with recommendations to: (1) ensure resources are available to adequately stage potentially resectable PDAC; (2) improve the quality of patient demographic information provided to MDTs in order to to make appropriate and accurate treatment pathway recommendations; (3) implement fast-track diagnosis and treatment pathways to minimise treatment delays; (4) prioritise PDAC patient access to palliative care support services both in hospitals and the community and (5) embrace research to improve outcomes of both early and advanced PDAC, given poor outcomes assosociated with this disease, irrespective of stage or interventions.
In summary, the CONTACT study reports a marked reduction in the staging and treatment provided to patients with PDAC diagnosed during the first wave of the UK COVID-19 pandemic. Though there was evidence of a change in pathway towards planned pre-operative chemotherapy prior to surgery among patients on a curative pathway during the pandemic, overall, this did not feed through to higher surgical resection rates. These data, essentially constituting a real-world experiment, strongly support the need now to prospectively assess the role of NAT and surgery in early PDAC. The overall findings that survival outcomes at 12 months were no different for patients whose standard pathways were modified compared with those whose pathways were not is a sobering signal that better treatments are urgently needed.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Mckay SC, Pathak S, Wilkin RJW, Kamarajah SK, Wigmore SJ, Rees J, et al. Impact of SARS-CoV-2 pandemic on pancreatic cancer services and treatment pathways: United Kingdom experience. HPB (Oxford). 2021;23:1656–65.
Rompianesi G, Shankar S, Reddy S, Silva M, Soonawalla Z, Friend PJ. Caught in the crossfire: hepato-bilio-pancreatic cancer surgery in the midst of COVID-19. Br J Surg. 2020;107:e309–10.
Collaborative C. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. 2020; Available from: www.bjs.co.uk.
Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J Am Med Assoc. 2020;323:1775–6. https://www.who.int/docs/default.
Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–7.
Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:180.
Yu J, Ouyang W, Chua MLK, Xie C. CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–10.
Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31:1088–9. https://doi.org/10.1016/j.annonc.2020.04.006.
Jones CM, Radhakrishna G, Aitken K, Bridgewater J, Corrie P, Eatock M, et al. Considerations for the treatment of pancreatic cancer during the COVID-19 pandemic: the UK consensus position. Br J Cancer. 2020;123:709–13. https://doi.org/10.1038/s41416-020-0980-x.
Curigliano G, Banerjee S, Cervantes A, Garassino MC, Garrido P, Girard N, et al. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol. 2020;31:1320–35.
NICE. Pancreatic cancer in adults: diagnosis and management. Pancreatic cancer in adults: diagnosis and management [Internet]. 2018;156:591–600. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29505215.
Catanese S, Pentheroudakis G, Douillard JY, Lordick F. ESMO management and treatment adapted recommendations in the COVID-19 era: Pancreatic Cancer. ESMO Open. 2020;5:e000804.
Lee LYW, Cazier JB, Starkey T, Briggs SEW, Arnold R, Bisht V, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–16.
Kleeff J, Korc M, Apte M, la Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:1–23. https://doi.org/10.1038/nrdp.2016.22.
Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333–48.
Gharaibeh A, Koppikar S J, Bonilla-Escobar F. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) in the International Journal of Medical Students. Int J Med Stud. 2014;2:36–7.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208.
Medical Research Council. Decision tool to decide whether or not your study is research as defined by the UK Policy Framework for Health and Social Care Research. NHS Health Research Authority. 2017; Available online: https://www.hra-decisiontools.org.uk/research/about.html.
Zhan HX, Xu JW, Wu D, Wu ZY, Wang L, Hu SY, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med. 2017;6:1201–19.
Altman AM, Wirth K, Marmor S, Lou E, Chang K, Hui JYC, et al. Completion of adjuvant chemotherapy after upfront surgical resection for pancreatic cancer is uncommon yet associated with improved survival. Ann Surg Oncol. 2019;26:4108–16.
Glasbey J, Ademuyiwa A, Adisa A, AlAmeer E, Arnaud AP, Ayasra F, et al. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol. 2021;22:1507–17.
Moslim MA, Hall MJ, Meyer JE, Reddy SS. Pancreatic cancer in the era of COVID-19 pandemic: Which one is the lesser of two evils? World J Clin Oncol. 2021;12:54–60.
Lee LYW, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–26.
Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38:1763–73.
Ghaneh P, Palmer D, Cicconi S, Halloran C, Psarelli E, Rawcliffe C, et al. Meeting abstract: ESPAC-5F: four-arm, prospective, multicenter, international randomized phase II trial of immediate surgery compared with neoadjuvant gemcitabine plus capecitabine (GEMCAP) or FOLFIRINOX or chemoradiotherapy (CRT) in patients with borderline resectable pancreatic cancer. J Clin Oncol. 2020;38:4505–05.
Mackay TM, Smits FJ, Roos D, Bonsing BA, Bosscha K, Busch OR, et al. The risk of not receiving adjuvant chemotherapy after resection of pancreatic ductal adenocarcinoma: a nationwide analysis. HPB. 2020;22:233–40.
Sata N, Kurashina K, Nagai H, Nagakawa T, Ishikawa O, Ohta T, et al. The effect of adjuvant and neoadjuvant chemo(radio)therapy on survival in 1,679 resected pancreatic carcinoma cases in Japan: Report of the national survey in the 34th annual meeting of Japanese Society of Pancreatic Surgery. J Hepatobiliary Pancreat Surg. 2009;16:485–92.
Mayo SC, Gilson MM, Herman JM, Cameron JL, Nathan H, Edil BH, et al. Management of patients with pancreatic adenocarcinoma: National trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. 2012;214:33–45.
Kagedan DJ, Dixon ME, Raju RS, Li Q, Elmi M, Shin E, et al. Predictors of adjuvant treatment for pancreatic adenocarcinoma at the population level. Curr Oncol. 2016;23:334–42.
Valle JW, Palmer D, Jackson R, Cox T, Neoptolemos JP, Ghaneh P, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: Ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32:504–12.
Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–24.
Baker G, Brom MK, Brown Z, Haldar D, Unit L, Harvey PR, et al. Receipt of curative resection or palliative care for hepatopancreaticobiliary tumours (RICOCHET): protocol for a nationwide collaborative observational study. JMIR Res Protoc. 2019;8:e13566.
The RICOCHET Study Group on behalf of the West Midlands Research Collaborative. Pancreatic enzyme replacement therapy in patients with pancreatic cancer: a national prospective study. 2021; Available from: https://doi.org/10.1016/j.pan.2021.05.299.
Acknowledgements
University of Birmingham, Pancreatic Society of Great Britain and Ireland (PSGBI), Association of Upper Gastrointestinal Surgery (AUGIS). With thanks to Mr. James Hodson for his expertise and advice on the statistical analysis for this study. Further thanks to Mr. Michael Walters and Mr. Terry Hughes, from the Birmingham Centre for Observational and Prospective Studies (BiCOPS), for their help in the delivery of this project.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Contributions
Members of the CONTACT Study are as follows: Steering Committee and Writing Group: All members of the steering committee contributed equally to study and protocol development. LH completed data analysis, prepared first draft of manuscript and edited, SM conceived idea for study, prepared first draft of manuscript and edited, JHS prepared first draft of manuscript and edited, JS coordinated communication between participating centres and edited manuscript, LG was our Patient and Care advocate, LM facilitated study commencement and edited the manuscript, TP facilitated study commencement and edited the manuscript, GR edited the manuscript, JV edited the manuscript, PC edited the manuscript and KJR conceived idea for study and edited the manuscript. Regional Leads: facilitated communications between steering committee and local sites and monitered data collection. Meta-Coordinators: distributed study information and updates to collaborators. Collaborators: completed data collection at local sites.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Patient consent not required for this study as only routinely collected datapoints were collected by the local team, and centrally analysed data was anonymous. The study was registered locally as a clinical audit or service evaluation at each participating site prior to patient identification and data collection.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hall, L.A., McKay, S.C., Halle-Smith, J. et al. The impact of the COVID-19 pandemic upon pancreatic cancer treatment (CONTACT Study): a UK national observational cohort study. Br J Cancer 128, 1922–1932 (2023). https://doi.org/10.1038/s41416-023-02220-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02220-2