Abstract
Glioma is a common malignant tumor of the central nervous system (CNS) that has no effective treatment. In this study, we report that colony-stimulating factor-1 receptor (CSF-1R) is a key mediator of malignant features in glioma via modulation of the activity of extracellular signal-regulated kinase 1/2 (ERK1/2) signaling. In general, CSF-1R upregulation in glioma is associated with poor histologic grade and sursvival. Enforced expression of CSF-1R is sufficient to enhance cell growth, migration, invasion, and epithelial–mesenchymal transition, while CSF-1R silencing suppresses the above-described malignant phenotypes. Mechanistic investigations show that CSF-1R promotes activation of the ERK1/2 signaling pathway. Inhibition of the ERK1/2 pathway by SCH772984 reduces CSF-1R-induced migration, invasion, and lung metastasis of glioma cells, thus establishing a role of the ERK1/2 signaling pathway in mediating the CSF-1R effect. In summary, our results suggest that CSF-1R overexpression in gliomas contributes to the malignant behaviors of cancer cells.
Similar content being viewed by others
Introduction
Glioma is by far the most common primary brain tumor in adults and one of the most common malignancies that threaten human health and increase morbidity [1, 2]. The average 5-year survival rate remains unsatisfactory in spite of aggressive treatment [3, 4]. The median survival of glioma patients is only 12–15 months [5]. A detailed study has been conducted to probe the pathogenesis of gliomas [6]. Nevertheless, the potential molecular network related to carcinogenesis as well as that related to the progression of glioma remains largely undefined.
Colony-stimulating factor-1 (CSF-1) is a cytokine that regulates the differentiation as well as the function of macrophages through its receptor, CSF-1 receptor (CSF-1R) [7, 8]. Ligand binding activates receptor kinases via oligomerization and transphosphorylation processes [9]. Cell variations occur at different phases of macrophages, which contribute to heterogeneity and variability of tumor-associated macrophages [10,11,12]. Bone marrow cells are characterized by plasticity, especially those of monocyte–macrophage lineage [13, 14]. The activated forms of macrophages called M1 and M2 are associated with deterministic growth factors in T helper 1 as well as T helper 2 cells [15, 16]. CSF-1 and CSF-2 (granulocyte–macrophage CSF) are commonly employed to stimulate mature macrophages, which also participate in the pro-inflammatory/antitumorigenic and anti-inflammatory/protumorigenic macrophage polarization, respectively [17,18,19].
In the current study, we found that CSF-1R expression was elevated in glioma. In addition, SCF-1R overexpression led to tumor growth as well as metastasis. Collectively, these outcomes shed novel light on the functional contributions of CSF-1R in glioma cells, thereby serving as a theoretical basis of exploring future therapeutic targets of CSF-1R.
Materials and methods
Cell culture
Glioma cell lines including U251 and U87 were obtained from American Type Culture Collection. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) in 5% CO2, 0.5% penicillin–streptomycin, and 1% glutamine at 37 °C. Cell lines were routine detection of contamination by PCR. SCH772984 was obtained from Santa Cruz Biotechnology.
Tissue array and immunohistochemistry
Two paraffin-embedded human glioma tissue microarrays containing the follow-up survival information were obtained from SuperBioChips (9995300A). Arrays were exposed to anti-CSF-1R antibody in 3% bovine serum albumin and 0.1% Triton X-100 (Sigma) at 4 °C for 24 h. UltraVision Quanto Detection System (Thermo Fisher Scientific Inc.) was employed. Diaminobenzidine was used for visualization of specific immunostaining, followed by counterstaining with hematoxylin (Sigma). Afterwards, two investigators who were blinded to the clinical characteristics independently evaluated the distribution and positive intensity of CSF-1R under a microscope. In brief, the scoring of staining was based on the proportion of positive tumor cells in tumor tissue (0, 0%; 1, <25%; 2, 25–50%; 3, 51–75%, and 4, >75%) and staining intensity (0, none; 1, weak; 2, moderate; and 3, strong). The following formula was applied for the staining index: staining intensity score × proportion of positive tumor cells (ranging from 0 to 12). A final score over 6 was considered as high expression.
Real-time PCR
RNeasy Mini Kit (Qiagen) was used to extract total RNA according to manufacturer’s instructions. Complementary DNA was synthesized from 1 μg of total RNA, using a Transcriptor First Strand cDNA Synthesis Kit (Roche) in the presence of both oligo (dT) and random primers. The comparative threshold cycle (Ct) method was utilized to assess the relative expression of each target gene after normalization to corresponding β-actin controls. The primers were as follows: CSF-1R, forward: 5′-CCTCGCTTCCAAGAATTGCA-3′ and reverse: 5′-CCCAATCTTGGCCACATGA-3′; β-actin, forward: 5′-CCTGGCACCCAGCACAATG-3′ and reverse: 5′-GGGCCGGACTCGTCATACT-3′.
Dox-inducible plasmid construction and transfection
CSF-1R cDNA (full-length) was subcloned into an inducible lentiviral pTINDLE vector provided by Xuewen Pan, which contained a transactivator (rtTA3) as well as an inducible promoter (pTRE-tight). CSF-1R short hairpin RNA and nonsilencing controls were cloned into pINDUCER vector. Lentiviral particles were generated by co-transfecting 293T cells with the lentiviral vector, pMD2.G (VSVG) (Addgene: 12259), pMDLg/pRRE (Addgene: 12251), and pRSV-REV (Addgene: 12253). Following lentivirus packaging, cells were infected with lentiviruses, which included the doxycycline (Dox)-inducible plasmid. Flow cytometry (FACSAria; BD Biosciences) was utilized to establish stable cell lines expressing shCSF-1R. Dox (Sigma-Aldrich) at 2 μg/ml and 200 ng/ml concentrations was employed to induce shCSF-1R and to express CSF-1R, respectively. To obtain inducible overexpression systems, CSF-1R expression was induced for 10 days before the stable lines were subjected to phenotypic analysis.
Western blotting
Western blotting was performed as described in a previous study [20, 21] with antibodies against CSF-1R (Santa Cruz Biotechnology, sc-118887), p27 (Santa Cruz Biotechnology, sc-71813), SKP2 (Santa Cruz Biotechnology, sc-74474), β-actin (Santa Cruz Biotechnology, sc-1615), ZO-1 (Santa Cruz Biotechnology, sc-33725), E-cadherin (Cell Signaling Technology, #14472), phosphorylated (p)-extracellular signal-regulated kinase (p-ERK) (Cell Signaling Technology, #4370), ERK (Cell Signaling Technology, #4695), p-AKT (Cell Signaling Technology, #4060), AKT (Cell Signaling Technology, #4691), vimentin (Cell Signaling Technology, #5741), p-RB1 (Cell Signaling Technology, #2181), RB1 (Cell Signaling Technology, #3590), cyclin D (Abcam, ab134175), cyclin E (Abcam, ab135380), p53 (Abcam, ab26), p21 (Abcam, ab109520), p16 (Abcam, ab51243), Ki67 (Abcam, ab16667), and proliferating cell nuclear antigen (PCNA) (Abcam, ab18197).
Cell viability and colony formation
Cells were seeded into 6-well plates with culture medium, followed by trypan blue exclusion assay to analyze viable cells at 0, 24, 48, and 72 h. To conduct anchorage-dependent colony formation assay, a total of 200 cells were cultured in 6-well plates, followed by incubation for 14 days. Subsequently, after fixation of the colonies in methanol, they were stained with 0.1% crystal violet (Sigma) for counting.
Cell growth assay
Cell growth curves were plotted using the Cell Counting Kit-8 assay (KeyGen Biotech, Nanjing, China) according to the manufacturer’s instructions. Cells were seeded in 96-well plates. After incubation with the CCK-8 reagent (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt) for 2 h, absorbance at 450 nm was measured using an Infinite M200 instrument (Tecan, Switzerland). Each sample was assessed in triplicate at each time point.
Cell migration and invasion assay
Transwell inserts for 24-well plate with uncoated porous filters (pore size, 8 μm) were used to examine cell migration, and Matrigel-coated porous filters (BD Biosciences) were used for determination of cell invasion. Briefly, 2 × 104 cells in 0.2 ml were seeded into inserts, which were cultured in serum-free DMEM, and 0.6 ml DMEM supplemented with 10% FBS was added to the lower part of the well. Cells that migrated to the other side of the filters were stained by crystal violet and further counted. At least three microscopic fields of each filter were counted to calculate the mean number of stained cells per field. Experimental procedures were conducted in triplicate.
Flow cytometry
For cell cycle analysis, after fixation in 75% ethanol, cells were stained with propidium iodide (Sigma). To perform cell apoptosis analysis, Annexin V-FITC Apoptosis Detection Kit (Abcam) was purchased to stain cells in accordance with the manufacturer’s instructions.
Tumor growth mouse models
The animal experiments were approved by the Committee for Animal Experimentation of China–Japan Union Hospital of Jilin University and were in line with the corresponding guidelines. In tumor growth analysis, 5 × 106 of U87 mock transfectants were subcutaneously injected into left flank, and equal number of CSF-1R transfectants was injected into the right side of nude mice. One week after cells were seeded, the mice were randomly divided into two groups (n = 6). Tumor volumes were monitored for 21 days. Tumor growth was monitored by calipers, and tumor volumes were calculated according to the formula ½ × length × width [2]. At the end of the experiment, tumors were resected, weighed, and analyzed by immunohistochemistry (IHC) and Western blotting (WB).
Statistical analysis
GraphPad Prism V was employed for statistical analysis. The Student’s t test was employed for statistical analyses, and a paired t test was utilized for analyzing paired U87 tumors. Two-sided Fisher's exact test was utilized to compare the correlation of CSF-1R expression with clinicopathologic characteristics. Kaplan–Meier analysis along with log-rank test was utilized for calculation of overall survival. P < 0.05 was considered as statistically significance.
Results
Expression of CSF-1R is frequently upregulated in glioma and correlates with poor survival
To explore the function of CSF-1R in glioma, the protein expression of CSF-1R was first assessed in glioma using tissue microarrays. CSF-1R expression levels were higher in patients with invasive glioma than in those in patients with noninvasive glioma and in benign tissue samples (Fig. 1a, b). Further, four paired tissue samples were randomly chosen to assess CSF-1R protein levels by WB, which revealed increased CSF-1R protein levels in glioma (Fig. 1c). Patients with recurrences of glioma exhibited higher mRNA expression of CSF-1R than did those without recurrences (Fig. 1d). In addition, mRNA expression of CSF-1R was much higher elevated in primary glioma tissues from metastatic subjects than in those without metastases (Fig. 1e). Kaplan–Meier survival analysis demonstrated a significantly reduced survival rate of glioma subjects harboring high CSF-1R expression compared to those with low CSF-1R expression (p = 0.026) (Fig. 1f). The above results suggest that expression of CSF-1R is upregulated in glioma and is also a biomarker of high histology grade and poor prognosis.
CSF-1R overexpression indicates poor prognosis and promotes glioma metastases. a Representative images of immunohistochemistry (IHC) staining of CSF-1R protein in glioma tissue microarrays. Scale bar, 50 μm. b IHC scores of CSF-1R staining in benign breast disease, noninvasive breast carcinoma, and invasive glioma tissues. Data were represented as means ± SD from three independent experiments. *P < 0.05; **P < 0.01. c CSF-1R protein level in glioma tissues (T) and paired normal tissues (N) were assessed by Western blotting. d Relative mRNA expression of CSF-1R in glioma patient samples with recurrence (n = 26) or without recurrence (n = 21) *P < 0.05. e Relative mRNA expression of CSF-1R in glioma patient samples with metastasis (n = 23) or without metastasis (n = 16) *P < 0.05. f Kaplan–Meier analysis of overall survival for patients with glioma. The analyses were conducted according to the immunohistochemistry of CSF-1R and the survival information provided by the supplier
CSF-1R overexpression promotes glioma growth, colony formation, migration, and invasion
To evaluate the role of CSF-1R in malignant phenotypes in glioma cells, cell growth, colony formation, and migration as well as invasion were assessed. To achieve CSF-1R overexpression, the coding region of SCF-1R was cloned into a Dox-inducible lentiviral vector, followed by subsequent transduction into U87 cells. Consequently, overexpression of CSF-1R enhanced cell growth (Fig. 2a, b). In addition, overexpression of CSF-1R induced PCNA and Ki67 expression in U87 cells (Fig. 2c). Consistently, overexpression of CSF-1R increased the number of anchorage-dependent colonies (Fig. 2d). Intriguingly, overexpression of CSF-1R dramatically promoted migration and invasion (Fig. 2e, f). Considering that cell invasion and morphological changes are tightly associated with epithelial–mesenchymal transition (EMT), WB was used to examine expression of epithelial markers, namely, E-cadherin as well as ZO-1, and mesenchymal markers, namely, vimentin, which revealed that overexpression of CSF-1R suppressed E-cadherin as well as ZO-1 expression, while increasing vimentin expression in U87 cells (Fig. 2g). These data indicate that overexpression of CSF-1R promoted cell growth, colony formation, migration, invasion, and EMT of glioma cells in vitro.
Overexpression of CSF-1R promotes migration and invasion in glioma cells. a CSF-1R modulated cell viability in vitro. Cell viability of vector and CSF-1R U87 cells was analyzed by trypan blue exclusion assays at different time points after Dox treatment. Data were represented as means ± SD from three independent experiments. *P < 0.05 **P < 0.01. b Cell growth was measured by CCK assay. Results were presented as means ± SD from three independent experiments. *P < 0.05. c PCNA and Ki67 expression were analyzed by Western blotting in control and CSF-1R-overexpressing cells. d Effects of CSF-1R on anchorage-dependent colony formation. Results were presented as means ± SD from three independent experiments. *P < 0.05. e CSF-1R regulated transwell cell migration and f Matrigel invasion. Overexpression of CSF-1R significantly enhanced migration and invasion in U87 cells. All results were represented as means ± SD from three independent experiments. *P < 0.05. g CSF-1R regulated epithelial–mesenchymal transition of glioma cells. Expression of epithelial markers, E-cadherin and ZO-1, and mesenchymal markers, vimentin, were analyzed by Western blotting. β-Actin was used as a loading control
CSF-1R knockdown suppresses glioma growth, colony formation, migration, and invasion
Next, we demonstrated that knockdown of CSF-1R mildly decreased cell growth and PCNA and Ki67 expression (Fig. 3a, c), and slightly decreased colony numbers (Fig. 3d). In addition, knockdown of CSF-1R significantly decreased migration and invasion (Fig. 3e, f). From the above-described results, it is clear that knockdown of CSF-1R increased E-cadherin as well as ZO-1 expression, but decreased vimentin expression in U251 cells (Fig. 3g). These data indicate that knockdown of CSF-1R suppressed glioma cell growth, colony formation, migration, and invasion, as well as EMT in vitro.
CSF-1R knockdown in glioma cells. a CSF-1R modulated cell viability in vitro. Cell viability of shcon and shCSF-1R U251 cells was analyzed by trypan blue exclusion assays at different times. Data were represented as means ± SD from three independent experiments. *P < 0.05; **P < 0.01. b Cell growth was measured by CCK assay. Results were presented as means ± SD from three independent experiments. *P < 0.05. c PCNA and Ki67 expression were analyzed by Western blotting in control and CSF-1R-knockdown cells. d Effects of CSF-1R on anchorage-dependent colony formation. Results were presented as means ± SD from three independent experiments. *P < 0.05. e CSF-1R regulated transwell cell migration and f Matrigel invasion. Knockdown of CSF-1R suppressed migration and invasion in U251 cells. All results were represented as means ± SD from three independent experiments. **P < 0.01. g CSF-1R regulated epithelial–mesenchymal transition of glioma cells. Expression of epithelial markers, E-cadherin and ZO-1, and mesenchymal markers, vimentin, were analyzed by Western blotting. β-Actin was used as a loading control
CSF-1R silencing promotes G1/S transition arrest and mediates apoptosis
To determine the effect of CSF-1R suppression on cell cycle, the DNA content after knockdown of CSF-1R in asynchronized U87 as well as U251 cells was detected by flow cytometry. CSF-1R knockdown promoted substantial G1-phase cell induction and S-phase cell reduction (Fig. 4a and S1A and S1B), indicating delayed cell cycle progression via the G1/S arrest. Moreover, CSF-1R suppression strongly induced apoptosis in U87 as well as U251 cells, as shown using Annexin V assay (Fig. 4b). In addition, WB analysis demonstrated sustained elevated cyclin E expression and decreased cyclin A expression following CSF-1R suppression (Fig. 4c), which indicated that the cells were blocked from entering the S phase.
CSF-1R inhibition results in impaired G1/S progression and induction of apoptosis. a Flow cytometry results showing cell cycle changes after CSF-1R knockdown in U87 and U251 cells. b Cell apoptosis assessed by Annexin V assay in asynchronized U87 and U251 cells following CSF-1R knockdown. Results were presented as means ± SD from three independent experiments. **P < 0.01. c Western blotting was used to examine the changes of key signaling molecules involved in G1/S cell cycle regulation
In addition, a remarkable enhancement of p27 protein level and reduced expression of SKP2, a key E3 ligase of p27 were detected (Fig. 4c). In brief, p27 suppresses the cyclin E/CDK2 and cyclin D/CDK4 complexes, which subsequently hinders cell cycle progression through the G1/S border. Therefore, the blocked G1/S transition might contribute to enhanced p27 expression. Moreover, reduced rRb (S807/S811) phosphorylation was detected following CSF-1R suppression. Considering the inhibitory role of p27 on cyclin E/CDK2 and cyclin D/CDK4, which are the upstream kinases of Rb, the enhanced p27 following CSF-1R suppression might inhibit Rb phosphorylation as well as subsequent E2F release.
ERK1/2 signaling mediates the function of CSF-1R in glioma
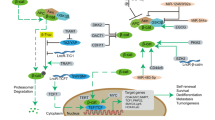
To determine the cell signaling variations following CSF-1R overexpression in U87 cells, WB was conducted on signaling molecule array in glioma. Intriguingly, AKT was not activated with CSF-1R overexpression (Fig. 5a), while CSF-1R overexpression markedly increased phosphorylation of ERK1/2.
CSF-1R enhances ERK1/2 signaling in glioma cells. a Effects of CSF-1R on ERK1/2 signaling. U87 stable transfectants were treated with Dox for 24 h. Indicated proteins were analyzed by Western blotting. b Effects of SCH772984 on CSF-1R-enhanced cell viability. Data were represented as means ± SD from three independent experiments. **P < 0.01. c Effects of SCH772984 on CSF-1R-enhanced cell migration and d invasion. Cells were treated with SCH772984 (5 mM) or DMSO during the migration and invasion assays. Data were represented as means ± SD from three independent experiments. **P < 0.01. e U87 cells were overexpressed with CSF-1R and then was treated with SCH772984 (5 mM). Expression of CSF-1R, E-cadherin, ZO-1, and mesenchymal markers, vimentin, were analyzed by Western blotting
To determine the effect of the ERK1/2 signaling pathway on CSF-1R-enhanced malignant phenotypes, glioma cells were exposed to SCH772984, an ERK1/2 inhibitor. We found that 5 μM SCH772984 significantly suppressed cell growth in CSF-1R-overexpressed cells (Fig. 5b). In contrast, transwell assays and WB results showed that CSF-1R-induced cell migration as well as invasion was significantly inhibited by ERK1/2 pathway blockage (Fig. 5c, e). These results suggested that CSF-1R could activate ERK1/2 pathway in glioma cells.
CSF-1R promotes glioma tumor growth in vivo
To confirm the role of CSF-1R in tumor growth in vivo, mock-treated and CSF-1R-overexpressing U87 cells were subcutaneously transplanted in mice. As a result, overexpression of CSF-1R significantly enhanced both tumor size and tumor weight (Fig. 6a). IHC of resected tumors was used to confirm CSF-1R expression in tissue (Fig. 6b). In addition, WB revealed that p-ERK1/2 expression was significantly increased in CSF-1R-overexpressing tumors (Fig. 6c). These results suggest that ERK1/2 pathway was involved in CSF-1R-enhanced tumor growth in vivo, indicating that CSF-1R promoted tumor growth of glioma cells by enhancing the activity of ERK1/2 pathway in vivo.
CSF-1R enhanced tumor growth and metastasis in vivo. a Effects of CSF-1R on glioma tumor growth in mouse model. U87 mock transfectants were subcutaneously injected into left rear flank of nude mice and equal number of CSF-1R-overexpressed cells were injected into the right rear flank of each mouse. The size of tumors was measured at indicated time points, and was shown as mean ± SD. Mice were sacrificed at day 21. Tumors were excised and weighted (right). *P < 0.05, n = 6. b Immunohistochemistry of transplanted tumors. Paraffin-embedded sections were immunostained with CSF-1R. Scale bar, 50 μm. c Analysis of indicated proteins expression in transplanted tumors by Western blotting
Discussion
Our findings demonstrate that upregulation of CSF-1R in glioma tissues is correlated with worse tumor grade and poor prognosis, consistent with previous studies [22, 23]. In addition, ectopic overexpression of CSF-1R in U87 cells remarkably enhances cell migration as well as invasion, while suppression of CSF-1R expression abolishes this effect, indicating the role of CSF-1R in increased invasion. In addition, we demonstrate that CSF-1R may participate in the ERK1/2 axis to enhance glioma cell invasiveness. More detailed studies are necessary to reveal the exact mechanisms of CSF-1R-driven cell invasiveness and the precise interaction of CSF-1R with ERK1/2 signaling pathway. Of note, glioma cells that harbor CSF-1R amplifications are likely to be prone to CSF-1R overexpression; therefore, CSF-1R suppression leads to strong growth inhibition as well as apoptosis induction [24].
Our mechanistic studies indicated that CSF-1R suppression decreases SKP2, increases p27, and blocks cell cycle via G1/S arrest. On the other hand, the primary tumor microenvironment is likely to provide additional signaling necessary for the function of endogenous CSF-1R, reflecting the limitations of ectopic expression in cell line models in exploration of the function of amplifying oncogenes [25, 26]. For instance, models might fail to harbor the microenvironment or genetic background necessary for the complete functions of endogenously amplifying oncogenes, which, hence, might not truly recapitulate the mechanism as well as phenotypes of oncogenes [27, 28].
Moreover, our findings indicate that CSF-1R suppression might harbor viable therapeutic value in tumors with high CSF-1R expression in vivo. As indicated by the outcomes of the xenograft, CSF-1R overexpression significantly enhanced tumor growth [29]. We acknowledge the shortcomings of the in vitro and in vivo cell line models in prediction of therapeutic values, owing to extremely simple clonal evolution of cultured cells in vitro as well as a lack of matrix interactions. Further studies are needed to further assess the therapeutic efficacy of CSF-1R suppression in patient-derived xenograft tumors and, ultimately, in glioma clinical trials. Collectively, our findings consider CSF-1R as an attractive target of cell cycle kinase for glioma cancers harboring CSF-1R amplifications.
In conclusion, our findings in this study suggest that CSF-1R may regulate ERK1/2 signaling, which is involved in CSF-1R-induced malignant behaviors of glioma cells. A comprehensive understanding of the effects and mechanisms of altered ERK1/2 signaling pathway may provide novel therapeutic strategies for glioma.
References
Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 2016;20 Suppl:S2–8.
Reifenberger G, Wirsching HG, Knobbe-Thomsen CB, Weller M. Advances in the molecular genetics of gliomas—implications for classification and therapy. Nat Rev Clin Oncol. 2017;14:434–52.
Coniglio SJ, Segall JE. Review: molecular mechanism of microglia stimulated glioblastoma invasion. Matrix Biol. 2013;32:372–80.
Urbanczyk H, Straczynska-Niemiec A, Glowacki G, Lange D, Miszczyk L. Case presentation—a five-year survival of the patient with glioblastoma brain tumor. Rep Pract Oncol Radiother. 2014;19:347–51.
Delgado-Lopez PD, Corrales-Garcia EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18:1062–71.
Noroxe DS, Poulsen HS, Lassen U. Hallmarks of glioblastoma: a systematic review. ESMO Open. 2016;1:e000144.
Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harbor Perspect Biol. 2014;6:a021857.
Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–20.
Hamilton JA, Cook AD, Tak PP. Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat Rev Drug Discov. 2016;16:53–70.
Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10:58.
Ao JY, Zhu XD, Chai ZT, Cai H, Zhang YY, Zhang KZ, et al. Colony-stimulating factor 1 receptor blockade inhibits tumor growth by altering the polarization of tumor-associated macrophages in hepatocellular carcinoma. Mol Cancer Ther. 2017;16:1544–54.
Cannarile MA, Ries CH, Hoves S, Ruttinger D. Targeting tumor-associated macrophages in cancer therapy and understanding their complexity. Oncoimmunology. 2014;3:e955356.
Weischenfeldt J, Porse B. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc. 2008;2008:pdbprot5080.
Mousa A, Cui C, Song A, Myneni VD, Sun H, Li JJ, et al. Transglutaminases factor XIII-A and TG2 regulate resorption, adipogenesis and plasma fibronectin homeostasis in bone and bone marrow. Cell Death Differ. 2017;24:844–54.
Muraille E, Leo O, Moser M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol. 2014;5:603.
Almatroodi SA, McDonald CF, Darby IA, Pouniotis DS. Characterization of M1/M2 tumour-associated macrophages (TAMs) and Th1/Th2 cytokine profiles in patients with NSCLC. Cancer Microenviron. 2016;9:1–11.
Sielska M, Przanowski P, Wylot B, Gabrusiewicz K, Maleszewska M, Kijewska M, et al. Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol. 2013;230:310–21.
Lou J, Low-Nam ST, Kerkvliet JG, Hoppe AD. Delivery of CSF-1R to the lumen of macropinosomes promotes its destruction in macrophages. J Cell Sci. 2014;127 Part 24:5228–39.
Wiehagen KR, Girgis NM, Yamada DH, Smith AA, Chan SR, Grewal IS, et al. Combination of CD40 agonism and CSF-1R blockade reconditions tumor-associated macrophages and drives potent antitumor immunity. Cancer Immunol Res. 2017;5:1109–21.
Tong J, Wang P, Tan S, Chen D, Nikolovska-Coleska Z, Zou F, et al. Mcl-1 degradation is required for targeted therapeutics to eradicate colon cancer cells. Cancer Res. 2017;77:2512–21.
Tong J, Tan S, Zou F, Yu J, Zhang L. FBW7 mutations mediate resistance of colorectal cancer to targeted therapies by blocking Mcl-1 degradation. Oncogene. 2017;36:787–96.
Yu YX, Wu HJ, Tan BX, Qiu C, Liu HZ. CSF-1R regulates non-small cell lung cancer cells dissemination through Wnt3a signaling. Am J Cancer Res. 2017;7:2144–56.
Huang L, Xu X, Hao Y, Chen J, Li L, Cheng J, et al. Overexpression of CSF-1R in nasopharyngeal carcinoma. Rev Roum Morphol Embryol. 2015;56:1279–83.
Cho MJ, Lee JY, Shin MG, Kim HJ, Choi YJ, Rho SB, et al. TSC-22 inhibits CSF-1R function and induces apoptosis in cervical cancer. Oncotarget. 2017;8:97990–8003.
Ryder M, Gild M, Hohl TM, Pamer E, Knauf J, Ghossein R, et al. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS ONE. 2013;8:e54302.
Huynh J, Kwa MQ, Cook AD, Hamilton JA, Scholz GM. CSF-1 receptor signalling from endosomes mediates the sustained activation of Erk1/2 and Akt in macrophages. Cell Signal. 2012;24:1753–61.
Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352:aad3018.
Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72.
Huang L, Xu X, Hao Y. The possible mechanisms of tumor progression via CSF-1/CSF-1R pathway activation. Rev Roum Morphol Embryol. 2014;55 Suppl:501–6.
Acknowledgements
We thank our lab members for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sun, L., Liang, H., Yu, W. et al. Increased invasive phenotype of CSF-1R expression in glioma cells via the ERK1/2 signaling pathway. Cancer Gene Ther 26, 136–144 (2019). https://doi.org/10.1038/s41417-018-0053-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-018-0053-y