Abstract
Decreased AMPK-eNOS bioavailability mediates the development of diabetic peripheral neuropathy (DPN) through increased apoptosis and decreased autophagy activity in relation to oxidative stress. Schwann cells are responsible for maintaining structural and functional integrity of neurons and for repairing damaged nerves. We evaluated the neuro-protective effect of cinacalcet on DPN by activating the AMPK-eNOS pathway using db/db mice and human Schwann cells (HSCs). Sciatic nerve of db/db mice was characterized by disorganized myelin, axonal shrinkage, and degeneration that were accompanied by marked fibrosis, inflammation, and apoptosis. These phenotypical alterations were significantly improved by cinacalcet treatment along with improvement in sensorimotor functional parameters. Cinacalcet demonstrated favorable effects through increased expression and activation of calcium-sensing receptor (CaSR)-CaMKKβ and phosphorylation of AMPK-eNOS signaling in diabetic sciatic nerve. Cinacalcet decreased apoptosis and increased autophagy activity in relation to decreased oxidative stress in HSCs cultured in high-glucose medium as well. This was accompanied by increased expression of the CaSR, intracellular Ca++ ([Ca++]i) levels, and CaMKKβ-LKB1-AMPK signaling pathway, resulting in the net effect of increased eNOS phosphorylation, NOx concentration, Bcl-2/Bax ratio, beclin 1, and LC3-II/LC3-I ratio. These results demonstrated that cinacalcet treatment ameliorates inflammation, apoptosis, and autophagy through increased expression of the CaSR, [Ca++]i levels and subsequent activation of CaMKKβ-LKB-1-AMPK-eNOS pathway in the sciatic nerve and HSCs under diabetic condition. Therefore, cinacalcet may play an important role in the restoration and amelioration of DPN by ameliorating apoptosis and improving autophagy.
Similar content being viewed by others
Introduction
Diabetic peripheral neuropathy (DPN) is one of the most common complications of diabetes in >50–60% of all diabetic patients, and it is also the leading cause of amputation worldwide1,2. The early changes in patients with DPN include accumulation of extracellular matrix proteins, inflammation, axonal degeneration, and loss of unmylelinated fibers, which cause sensorimotor conduction delays and irreversible nerve damage. It is well known that hyperglycemia plays a main role in DPN3,4,5,6,7 with regard to the changes in oxidative–nitrosative stress, neuro-inflammation, mitochondrial dysfunction, bio-energetic crisis, and demyelination7.
Schwann cells (SCs) are specialized glial cells in the peripheral nervous system that are responsible for maintaining structural and functional integrity of neurons and for repairing damaged nerves8,9. Hyperglycemia-induced SC damages may reduce nerve conduction velocity, accelerate axonal atrophy, and impair axonal regeneration10. Moreover, hyperglycemia-induced SC damages include such morphological changes as swelling and vacuolization that result in the destruction of organelles. Clearance of defective organelles constitutes the very core of the autophagy process that is an important physiological and defensive mechanism of the cell and body under such deranged metabolic conditions as nutrient or energy excess and deprivation11. Chronic hyperglycemia with diabetes impairs cellular autophagy and exacerbates apoptosis associated with DPN7,12. Autophagy promotes cell survival by sequestering senescent or damaged organelles/proteins in autophagosomes for recycling of their products11. Therefore, an enhancement of autophagy and a concomitant suppression of apoptosis of SCs might be the optimal strategy for the prevention and regression of DPN.
AMP-activated protein kinase (AMPK) is a master controller of cellular energy balance that activates catabolic pathways in state of energy deprivation13. Chronic nutrient excess state associated with prolonged diabetes triggers a switching off of AMPK, which results in impaired peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) activity and diminished mitochondrial14 and endothelial nitric oxide synthase (eNOS) activities15, leading to neurodegeneration in patients with DPN. The important mode of AMPK activation relies on phosphorylation at the 172nd threonine residue of the α-subunit by upstream kinases, including Ca++/calmodulin-dependent protein kinase kinase β (CaMKKβ) and liver kinase B1 (LKB1). The LKB1 forms a complex with STRAD and MO25 in response to an elevation in AMP/ATP ratio16, which phosphorylates the AMPKα subunit to trigger the AMPK pathway. CaMKKβ is an alternative upstream kinase of AMPK that responds to the change in intracellular Ca++ ([Ca++]i) concentration. Elevated [Ca++]i increases the activity of AMPK, independent of the adenylate energy balance17.
The calcimimetic, (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet), devised originally for the treatment of secondary hyperparathyroidism, exerts its effect by stimulating Ca++-sensing receptor (CaSR) mainly in the parathyroid glands18. Activated upon Ca++ ions, the expression of cellular surface CaSR is crucial for maintaining a stable serum Ca++, which is achieved primarily through the regulation of parathyroid hormone secretion and renal Ca++ excretion. Interestingly, the expression of CaSR has been demonstrated in the vasculature and perivascular sensory nerves18,19. CaSR activation by cinacalcet is known to activate CaMMK-LKB1-AMPK pathway. Activation of AMPK and LKB-1 is crucial for SC-mediated axonal maintenance while LKB deletion is responsible for axonal degeneration20. Moreover, the CaSR is known to modulate cell proliferation and apoptosis and coordinate oncogene expression, chemotaxis, and autophagy. Exposed under constant metabolic stress, these axons and SCs are prone to mitochondrial dysfunction featuring derangements in [Ca++]i homeostasis and associated downstream signaling that are key causal factors for the development of DPN, making it an ideal therapeutic target at the same time21.
To date, there is no curative therapy currently available to deter the progression of DPN; only a handful of studies have demonstrated improvements in indices of neuropathy through the activation of AMPK in cultured neurons22 and peripheral nerves of type 1 diabetic rats23. On this account, we assumed that cinacalcet treatment may modulate DPN activity through the axis delineated in the previous study. Thus we investigated the protective and/or reversal effect of cinacalcet against neural glucotoxicity through the changes in the AMPK-eNOS pathway in the sciatic nerve of db/db mice and human Schwann cells (HSCs).
Materials and methods
Experimental animals and assessment of peripheral nerve function
All animal experiments were performed in accordance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee (IACUC) at College of Medicine, the Catholic University of Korea (CUMC-2014−0165-01). Eight-week-old male C57BLKS/J db/m and db/db mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and db/m and db/db mice were divided into four groups. Cinacalcet (10 mg/kg) mixed into standard chow diet or a regular diet was administered to db/db mice (db/db+cina; n = 8) and age- and gender-matched db/m mice (db/m+cina; n = 8) for 12 weeks starting at 8 weeks of age.
After 12 weeks of cinacalcet treatment, we performed electrophysiological and sensory threshold tests in the following order: tactile responses (a response 50% of the times the tip is applied to the hind paw) to stimulation using flexible von Frey filaments and then sciatic motor nerve conduction latency (MNCL), as described previously15. After the tests were completed, blood was collected from the left ventricle and the plasma was stored at −70 °C for subsequent analyses, and we collected the sciatic nerves under general anesthesia with 10 mg/kg xylazine hydrochloride (Rompun; Bayer, Leuverkusen, Germany) and 30 mg/kg tiletamine plus zolazepam (Zoletil; Virbac, Carros, France). Some sciatic nerve samples were fixed in normal buffered 4% formalin for immunohistochemistry, and the others were stored in a solution for electron microscopy. We also collected the sciatic nerves in 8-week old male db/m and db/db mice for evaluation of cinacalcet effect on the recovery of DPN.
Assessment of blood glucose, HbA1c, plasma ionized calcium, and PO4 − concentrations
After 12 weeks of treatment with cinacalcet, blood glucose was measured using an Accucheck meter (Roche Diagnostics, St. Louis, MO). Hemoglobin A1c (HbA1c) was determined on red cell lysates using high-performance liquid chromatography (BioRad, Hercules, CA). Plasma ionized calcium (iCa++) and PO4− concentrations were measured using colorimetry (Samkwang Medical Laboratory, Seoul, Korea).
Light and electron microscopic analysis
Nerve morphology
Sciatic nerve samples were fixed in 4% paraformaldehyde. Trichrome-stained nerves were used to examine the effect of cinacalcet on nerve fibrosis. Ten consecutive nerve cross-sections were photographed using a digital camera (Olympus DP11; Olympus America, Melville, NY) by an examiner who was blinded to the tissue source. Each nerve section was sampled in a serpentine pattern such that the entire nerve section was analyzed with no overlapping fields. We performed immunohistochemistry for type IV collagen (Col IV; Biodesign International, Saco, ME, USA) and 8-hydroxy-deoxyguanosine (8-OH-dG; JalCA, Fukuroi, Shizuoka, Japan), an oxidative DNA damage marker.
Immunofluorescence double staining for F4/80 and TdT-mediated dUTP-biotin nick end labeling (TUNEL) and SOX10, β3-tubulin, and LC3
For immunofluorescence double staining, apoptosis was detected by the ApopTag Fluorescein In Situ Apoptosis Detection Kit (S7110; Chemicon International, Temecula, CA), as described previously15. And then, sections were incubated overnight with cell surface glycoprotein F4/80 (Serotec, Oxford, UK) and a Texas red-labeled secondary antibody and counterstained with 4,6-diamidino-2-phenylindole (DAPI). We also performed immunofluorescence double staining for SOX10 (Abcam, Cambridge, UK), β3-tubulin (1:50; Cell Signaling Technology, Danvers, MA), and LC3 (1:200; Sigma-Aldrich, St. Louis, MO, USA). The fluorescent images were examined under a laser scanning confocal microscope system (Carl Zeiss LSM 700, Oberkochen, Germany).
Electron microscopy
For transmission electron microscopy (TEM), sciatic nerves specimens were fixed in 4% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate buffer overnight at 4 °C. After washing in 0.1 M phosphate buffer, the specimens were post-fixed with 1% osmium tetroxide in the same buffer for 1 h. The specimens were then dehydrated using a series of graded ethanol, exchanged through acetone, and embedded in Epon 812. Ultrathin sections (70–80 nm) were obtained by ultramicrotome (Leica Ultracut UCT, Leica, Germany) and were double stained with uranyl acetate and lead citrate and examined in transmission electron microscope (JEM 1010, Tokyo, Japan) at 60 kV. We measured areas of unmyelinated fiber, axonal diameters, G ratio, and the number of degenerative fiber using NIH Image J.
Western blot analysis
The total proteins of the sciatic nerve tissues were extracted with a Pro-Prep Protein Extraction Solution (Intron Biotechnology, Gyeonggi-Do, Korea), following the manufacturer’s instructions. Western blot assay was performed with specific antibodies for CaSR (Thermo Fisher Scientific Inc, Waltham, MA, USA), CaMKKβ (Santa Cruz Biotechnology, Santa Cruz, CA, USA), total LKB1 (Cell Signaling Technology, Danvers, MA, USA), phosphor-Ser428 LKB1 (Cell Signaling Technology, Danvers, MA, USA), total AMPK (Cell Signaling Technology, Danvers, MA, USA), phospho-Thr172 AMPK (1:2000; Cell Signaling Technology, Danvers, MA, USA), total eNOS (Cell Signaling Technology, Danvers, MA, USA), phospho-Ser1177 eNOS (Cell Signaling Technology, Danvers, MA, USA), PGC-1α (Novus Biologicals, Littleton, CO, USA), B cell leukemia/lymphoma 2 (Bcl-2) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), BCL-2-associated X protein (Bax) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), beclin-1 (Novus Biologicals, Littleton, CO), and LC-3 (Sigma-Aldrich, St. Louis, MO). After incubation with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (Cell Signaling Technology, Danvers, MA), target proteins were visualized by an enhanced chemiluminescence substrate (ECL Plus, GE Healthcare Bio-Sciences, Piscataway, NJ).
HSC culture study
[Ca++]i measurement
HSCs were cultured in SC Medium (ScienCell Research Laboratories, San Diego, CA), as described previously15. Passages 4–8 were used in all experiments. The HSCs were exposed to low glucose (5 mmol/L d-glucose; low-glucose) or high glucose (40 mmol/L d-glucose), with or without the additional 24-h application of cinacalcet (15 and 100 nM). Calcium concentrations were determined based on the ratio of fura-2 fluorescence intensities at 340-nm excitation and 380-nm excitation. The ratio of 340/380 directly reflects the amount of [Ca++]i. The 340-nm fluorescence of fura-2 increases and the 380-nm of fura-2 decreases with increasing [Ca++]i. For [Ca++]i measurements, HSCs (20,000 cells/well) were plated on black 96-well plates with a clear bottom in complete medium. After 1 day, the cultures were serum-starved for 2 h in SC media. In the last 45 min of serum starvation, 5 mM FURA-2AM without Ca++ was added to the cells and then rinsed with Hank's Balanced Salt Solution (GibcoBRL, Grand Island, NY). FURA-2AM-loaded cells were sequentially excited at 340 and 380 nm by spectrophotometer microplate reader (Synergy MX; BioTek, Winooski, VT).
Immunofluorescence and western blot analyses in the HSCs
To evaluate the effects of cinacalcet on CsSR, CaMKKβ, phospho-Ser428 LKB1, and phospho-Thr172 AMPK expression, we performed immunofluorescence analysis with specific antibodies for CaSR, CaMKKβ, phospho-Ser428 LKB1, and phospho-Thr172 AMPK by using tyramide signal amplification fluorescence system and counterstained with DAPI. In addition, the total proteins of the HSCs were extracted with a Pro-Prep Protein Extraction Solution, following the manufacturer’s instructions. After incubation with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (Cell Signaling Technology, Danvers, MA), target proteins were visualized by an enhanced chemiluminescence substrate (ECL Plus, GE, Healthcare Bio-Science, Piscataway, NJ). To evaluate the anti-apoptotic effects of cinacalcet on HSCs in high-glucose medium, the number of TUNEL-positive cells was counted in 10 randomly chosen fields at a magnification of ×400. We also measured the concentration of NOx to quantify NO production in cell-culture media. The total NO3 and NO2 were quantified using the Nitric Oxide Assay Kit (Bio Vision, Mountain View, CA).
HSCs with small interfering RNA (siRNA) transfection
siRNAs, targeted against CaMKKβ, LKB1, and scrambled siRNA (siRNA cont), were complexed with the transfection reagent (Lipofectamine 2000; Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. The sequences of the siRNAs are as follows: CaMKKβ, 5′-GGAUCUGAUCAAAGGCAUCTT-3′; LKB1, 5′-GGACUGACGUGUAGAACAATT-3′; and nonspecific scrambled siRNA, 5′-CCUACGCCACCAAUUUCGU-3′ (Bioneer, Daejeon, Korea). HSCs in 6-well plates were transfected with a final concentration of 50 nM CaMKKβ and LKB1 siRNAs for 24 h using the transfection reagent (Lipofectamine 2000) in Opti-MEM media (Gibco Life Technologies), according to the manufacturer’s instructions. After transfection, cells were treated with cinacalcet (5 nM) in high-glucose medium to evaluate the effects of siRNAs on HSC reactions.
Data analysis
SPSS version 16 (SPSS. Inc., Chicago, IL) was used to conduct the statistical analysis. Group differences were evaluated using an analysis of variance with the Bonferroni correction. Non-normally distributed data were analyzed by Mann–Whitney U test. The results are expressed as mean ± SD. A P value of <0.05 was considered statistically significant.
Results
Body weight, blood HbA1c, glucose, iCa++, and PO4 3 − levels
The body weights of db/db mice were greater than those of db/m mice in both the cinacalcet treatment and control groups at the end of the study (p < 0.001, Table 1). No changes in body weight were noted in db/m and db/db mice following the 12-week treatment with cinacalcet. HbA1c and fasting blood sugar concentrations were significantly higher in db/db mice than those in db/m mice in both the cinacalcet treatment and control groups (p < 0.001, Table 1). Interestingly, cinacalcet treatment did not change fasting blood glucose, HbA1c, or serum iCa++ and PO43− concentrations in db/db or db/m mice.
Assessment of peripheral nerve function
Tactile response thresholds, motor nerve conduction latency, and action potential amplitude in the sciatic nerve
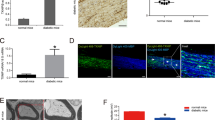
Tactile response thresholds (Fig. 1a, 1.09 ± 0.28 vs. 0.0.53 ± 0.23 g, p < 0.001) and MNCL (Fig. 1b, 1.81 ± 0.06 vs. 1.51 ± 0.05 ms, p < 0.01) were increased in db/db cont compared with those in db/m cont mice at the end of the 12-week study. Interestingly, cinacalcet treatment significantly improved the tactile response threshold and MNCL in db/db mice compared to those in db/db control (Fig. 1a, 0.74 ± 0.18 vs. 0.36 ± 0.14 g, p < 0.01 and Fig. 1b, 1.64 ± 0.05, vs. 1.48 ± 0.06 ms, p < 0.01, respectively) to the levels similar to those in db/m mice. Consistent with the improvement in the tactile response threshold and MNCL, cinacalcet treatment increased action potential amplitude in db/db mice (Fig. 1c, 873 ± 224 vs. 1199 ± 124 μm, p < 0.01). However, there were no changes in the tactile response, sciatic motor conduction latency, and action potential amplitude in the db/m mice treated with or without cinacalcet.
a–c Effects of cinacalcet on the tactile threshold (a), motor conduction latency (b), and action potential amplitude (c) were determined at 20 weeks in db/m and db/db mice with or without cinacalcet treatment. (n = 8 in each groups) *p < 0.05, **p < 0.01, and #p < 0.001 compared with the other groups
Assessment of nerve pathology
Expressions of nerve fibrosis and Collagen IV (Col IV)
The sciatic nerve in the db/db cont mice showed significant nerve fibrosis as reflected by increased trichrome-stained area when compared with that of db/m mice (Fig. 2a, b, 8.3 ± 2.1 vs. 3.4 ± 1.6%, p < 0.01) Immunohistochemical staining revealed increased expression of Col IV in the sciatic nerve of db/db cont compared to that of db/m cont mice (Fig. 2a, b, 2.4 ± 0.5 vs. 1.0 ± 0.5 folds, p < 0.01). Increased expression of Col IV and consistent increase in the extent of fibrotic area in the sciatic nerve of db/db control mice were improved to the levels similar to those of db/m mice with cinacalcet treatment (Fig. 2a, b, 3.8 ± 1.7% and 1.2 ± 0.4 folds, p < 0.01, respectively). Thus a 12-week cinacalcet treatment significantly improved fibrosis in the sciatic nerve of db/db mice.
a–d. Nerve fibrosis, oxidative stress, inflammatory cell infiltration, and apoptosis in the sciatic nerves were determined at 20 weeks in db/m and db/db mice with or without cinacalcet treatment. Representative Masson’s trichrome staining and immunohistochemical staining for Col IV (a, b) and 8-hydroxy-deoxyguanosine (8-OH-dG), immunofluorescence for TUNEL, F4/80-positive cells, TUNEL-SOX10- and TUNEL-β3 tubulin-positive cells, and LC3-SOX10- and LC3-β3 tubulin-positive cells were determined (b). The white dotted line box indicates the area for each enlarged figure. The white arrows indicate TUNEL-SOX10- and TUNEL-β3 tubulin-positive cells and LC3-SOX10- and LC3-β3 tubulin-positive cells. The quantitative analyses of the results are shown (d, original magnification, ×1000). Scale bar = 10 μm (a, c). (n = 8 in each groups) **p < 0.01, and #p < 0.001 compared with the other groups
8-OH-dG, F4/80-positive cells, and TUNEL-positive cells in the sciatic nerve
The presence of 8-OH-dG-positive area was more prominent in db/db cont mice compared with that in db/m cont mice, reflecting increased amount of neuronal oxidative stress (Fig. 2a). However, cinacalcet treatment decreased the production of 8-OH-dG to the level comparable to that of db/m mice (Fig. 2b). We observed the number of TUNEL-positive neural cells, including SC and peripheral neuronal cells which are marked with SOX10 and β3-tubulin, respectively. Expression of TUNEL-positive cells was significantly increased in db/db cont compared with that in db/m cont mice (Fig. 2c, d, 25 ± 6 vs. 13 ± 3 cells/high-power field (HPF), p < 0.01). Cinacalcet treatment reduced the number of TUNEL-positive neural cells in db/db mice (12 ± 1.7 cells/HPF). Additionally, F4/80-positive inflammatory cell infiltration was severe in db/db mice when compared with that in non-diabetic db/m mice (2.1 ± 0.5 vs. 0.2 ± 0.4 cells/HPF, p < 0.01). Cinacalcet treatment reduced the number of F4/80-positive cells in db/db mice (0.2 ± 0.3 cells/HPF). There were no significant changes in the expression of TUNEL- and F4/80-positive cells in the sciatic nerve of non-diabetic mice with or without cinacalcet treatment. Significant decrease in the expression of LC3 in SOX10- and β3-tubulin-positive cells in diabetic control mice increased with cinacalcet treatment to the levels comparable to those of non-diabetic mice, reflecting the recovery of autophagy process (Fig. 2c, d).
Electron microscopy
The ultrastructural features of sciatic nerves in db/db mice were characterized by severe myelin disruption with axonal shrinkage, degenerated SCs, and decreased amount of unmyelinated fibers with vacuolization when compared with those in db/m cont mice (Fig. 3a). Sciatic nerves from the db/db mice treated with cinacalcet displayed ultra-micro structures that resembled those from db/m control mice. Significant decreases in both axonal diameter and area and unmyelinated area of db/db mice were increased with cinacalcet treatment to the levels comparable to those of non-diabetic mice groups (p < 0.05 and p < 0.01, respectively; Fig. 3b). In contrast, increased G ratio in db/db mice was decreased with cinacalcet treatment, indicating optimized axonal myelination24 with improved functional and structural indices (p < 0.01; Fig. 3b).
a, b The axonal area, area of unmyelinated fiber, and Schwann cell degeneration in the sciatic nerves were determined at 20 weeks in db/m and db/db mice. Representative electron microscopic images of the sciatic nerve bundles (×5000) (a). Marked decreases in the number of unmyelinated nerve bundles (open arrows) and prominent axonal degeneration (arrows) were observed in the db/db controls. These deficits were improved by the 12-week cinacalcet treatment in the db/db+cina mice (a). Quantitative analyses of axonal area and areas of unmyelinated fiber are shown (b). Scale bar = 2 μm (a). (n = 8 in each groups) *p < 0.05, **p < 0.01compared with the other groups
Expression levels of CaSR, CaMKKβ, phospho-Ser428 and total LKB1, phospho-Thr172 and total AMPK expression, PGC-1α, phospho-Ser1177 eNOS, Bcl-2, Bax, Beclin-1, and LC3-1 and -II in the sciatic nerve
On western blot analysis, CaSR, CaMKKβ, phospho-LKB1, phospho-AMPK, PGC-1α, and phospho-eNOS expression was significantly decreased in the sciatic nerve of db/db cont compared with that of db/m cont mice (Fig. 4a, b). These findings suggest that diabetes itself decreases the activation of CaSR-CaMKKβ and LKB1 phosphorylation, resulting in a decrease in AMPK phosphorylation, which seems to be related to the development of diabetic peripheral nerve damage. In contrast, cinacalcet treatment increased the expression of CaSR, CaMKKβ, phospho-Ser428 LKB1, and phospho-Thr172 AMPK, which subsequently recovered PGC-1α and eNOS expression in the sciatic nerve of db/db mice (Fig. 4a, b). For further evaluation of apoptosis and autophagy, we also measured the expression of Bcl-2/Bax ratio, Beclin-1, and LC3-II/LC3-I ratio in the sciatic nerves. Significant decreases in the expression of Bcl-2/Bax, Beclin-1, and LC3-II/LC3-I ratio in the sciatic nerve of db/db mice increased with cinacalcet treatment to the levels comparable to those of db/m mice (Fig. 4c, d).
a, b The expression levels of CaSR, CaMKKβ, phospho-Ser428 LKB1, phospho-Thr172 AMPK, PGC-1α, phospho-Ser1177 eNOS, Bcl-1, Bax, Beclin-1, LC3-I, and LC3-II of the sciatic nerve were determined at 20 weeks in db/m and db/db mice. Cinacalcet treatment upregulates the expression of these intracellular signal pathway after the 12-week treatment in db/db mice. Representative western blot of CaSR, CaMKKβ, phospho-Ser428 LKB1, phospho-Thr172 AMPK, PGC-1α, phospho-Ser1177 eNOS, and β-actin (a) and quantitative analyses of the results are shown (b). c, d Representative western blot of Bcl-2 and Bax (c) and Beclin-1, LC3-I, and LC3-II (d) and their quantitative analyses of the results are shown (c, d, respectively). (n = 2 in each groups) *p < 0.05, **p < 0.01, and #p < 0.001 compared with the other groups
Effects of cinacalcet on [Ca++]i in HSCs
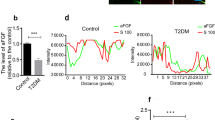
It is well known that an increase in [Ca++]i is associated with the activation of CaMKK and LKB1, which are potent activators of AMPK. Therefore, we measured the effect of cinacalcet on [Ca++]i in the HSCs grown in either low- or high-glucose medium with or without cinacalcet. Interestingly, both 15 and 100 nM of cinacalcet significantly increased the peak [Ca++]i levels and its area under curve (Fig. 5a, b). We further evaluated the effects of cinacalcet on AMPK activation in cultured HSCs by immunofluorescence staining and western blot analysis. The intracellular LKB-1-AMPK-PGC-1α-eNOS signaling pathway is thought to play an important role in the maintenance of normal SC function. Thus we investigated the upstream signals of AMPK; significant decreases in CaSR, CaMKKβ, phospho-Ser428 LKB1, and phospho-Thr172 AMPK expression were noted in HSCs grown in high-glucose medium, resulting in subsequent decreases in PGC-1α and phospho-Ser1177eNOS expression, which are downstream targets of AMPK (Fig. 6a, b). Consistent with the intracellular signaling changes, decreased extracellular NOx concentration and Bcl-2/Bax ratio in high-glucose medium was increased significantly with cinacalcet treatment with subsequent decrease in TUNEL-positive cells in high-glucose medium (Fig. 6b−d). Such changes were not observed either in low-glucose medium or in osmotic control (not shown), suggesting that high-glucose medium takes part in the suppression of [Ca++]i-CaMKKβ-LKB-1-phospho-AMPK signaling and subsequent PGC-1α-phospho-eNOS-NOx axis in HSCs, which resulted in increased apoptotic cell deaths of HSCs. We also evaluated whether AMPK phosphorylation by cinacalcet preserves autophagy activity in SCs. The high-glucose-induced decreases in beclin-1, LC3-II/LC3-I ratio, and number of LC3 punctate in SCs were completely recovered by cinacalcet treatment to the levels similar to those of HSCs grown in low-glucose medium (Fig. 6e).
a, b. To determine whether the addition of cinacalcet might modulate [Ca++]i in HSCs, FURA-2AM-loaded human Schwann cells (HSCs) were stimulated using different concentrations (15, 100 nM) of cinacalcet in low-glucose (LG; 5 mmol/l d-glucose) or high-glucose (HG; 40 mmol/l d-glucose) medium. The area under curve (AUC) was estimated from the baseline of normalized data (at the point of injection) to a fluorescence level and between time points of injection (0 min) and 10 min. The peak of the curve was measured as the highest value of the curve. The peak amplitude and AUC of [Ca++]i were significantly increased by cinacalcet in dose-dependent manners (a, b) in both LG and HG media. In a, the arrow denotes the administration of cinacalcet (15 and 100 nM, respectively). (n = 6 independent experiments in each experiments). *p < 0.05, **p < 0.01, and #p < 0.001 compared with the other groups
a Immunofluorescence analysis was performed for CaSR, CaMKKβ, and phosphor-Ser428 LKB1 in the HSCs with or without cinacalcet treatment (15 nM; original magnification, ×400) and the quantitative analyses of the results are shown. Scale bars represent 30 μm. (n = 6 independent experiments in each experiments) (a). b, c The effect of cinacalcet on intracellular signals and apoptosis in the human Schwann cells (HSCs) cultured in low-glucose (LG; 5 mmol/l d-glucose) or high-glucose (HG; 40 mmol/l d-glucose) conditions without or with dose-dependent cinacalcet treatment (1, 5, 15 nM) were determined. CaSR, CaMKKα/β, total LKB1, phosphor-Ser428 LKB1, total AMPK, phospho-Thr172 AMPK, PGC-1α, phospho-Ser1177 eNOS, NOx, BCL-2, BAX, and β-actin levels were assessed in the cultured HSCs. Representative western blot analyses and quantitative analyses of CaSR, CaMKKα/β, total LKB1, phospho-Ser428 LKB1, total AMPK, phospho-Thr172 AMPK, PGC-1α, and phospho-Ser1177 eNOS are shown (b). The NOx concentrations from the supernatant of HSCs are shown (b). Representative western blot analyses and quantitative analyses of BCL-2 and BAX are shown (c). d, e. Marked increases in TUNEL-positive HSCs were observed in the HG medium compared to HG+15 group. The quantitative analyses of the results are shown (d). Representative western blot analyses and quantitative analyses of Beclin-1, LC3-1, and LC3-II are shown (e). The quantitative analyses of the results are shown (e). Marked decreases in the number of punctate in an SC were observed in the HG medium compared to HG+15 group (e). (n = 4 independent experiments in each experiments) *p < 0.05, **p < 0.01, and #p < 0.001 compared with the other groups
Transfection of HSCs with CaMKKβ and LKB1 siRNAs suppressed the expression of CaMKKβ and phospho-Ser428 LKB1 by 80% and 60%, respectively, in low-glucose medium (Fig. 7a). Transfection with either CaMKKβ or LKB1 siRNA resulted in the dual suppression in the expression of CaMKKβ and phospho-LKB1 despite cinacalcet treatment. Moreover, Cinacalcet treatment did not increase the expression of phospho-Thr172 AMPK-PGC-1α-phospho-Ser1177 eNOS-NOx signaling in HSCs when transfected with either CaMKKβ or LKB1 siRNA (Fig. 7b, c).
a, b The cultured HSCs were transfected with a final concentration of 50 nM CaMKKβ and LKB1 siRNAs for 24 h by transfection reagent in low-glucose medium. Representative western blot analyses of CaMKKβ and phospho-Ser428 LKB1 and β-actin levels and the quantitative analyses of the results are also shown (a). **p < 0.01 compared with control siRNA. The cultured HSCs were transfected with a concentration of 50 nM CaMKKβ and LKB1siRNAs, respectively, for 24 h by transfection reagent and treated with cinacalcet (5 nM) in high-glucose medium, as well as CaMKKβ, phosphor-Ser428 LKB1, total AMPK, phospho-Thr172 AMPK, PGC-1α, phospho-Ser1177 eNOS, and β-actin levels and the quantitative analyses of the results are also shown (b). The NOx concentrations from the supernatant of HSCs are also shown (b). (n = 4 independent experiments in each experiments) *p < 0.05, **p < 0.01 compared with the other groups
Assessment of sciatic nerve function and phenotypes in 8-week-old db/m and db/db mice
To evaluate the effect of cinacalcet on the prevention and restoration of DPN, we investigated the functional and phenotypic changes in the sciatic nerves of 8-week-old db/m and db/db mice before the treatment. While tactile threshold was significantly increased in the sciatic nerve of db/db mice (p < 0.05), there were no significant differences in motor conduction latency and action potential amplitude between that of db/m and db/db mice (Fig. 8a). However, area of fibrosis, oxidative stress, and neuronal degeneration including decreased axonal diameter and area were prominent in the sciatic nerve of db/db mice with increased G ratio and decreased area for unmyelinated fibers (Fig. 8b). Furthermore, increased expression of F4/80- and TUNEL-positive cells and decreased expression of LC3-positive cells were noted in the sciatic nerve of db/db mice compared with those in db/m mice (Fig. 8c) and these changes were in line with decreased expression of CaSR-AMPK-eNOS signaling pathway in the same group (Fig. 8d).
a Effects of cinacalcet on the tactile threshold, motor conduction latency, and action potential amplitude were determined. b Nerve fibrosis (Masson’s trichrome and Col IV), oxidative stress (8-OH-dG), and the axonal diameter and area, the G ratio, and area of unmyelinated fiber in the sciatic nerves were determined. Representative electron microscopic images of the sciatic nerve bundles (×5000) are shown. Scale bars represent 2 μm. c Immunofluorescences for TUNEL, F4/80-positive cells, TUNEL-SOX10- and TUNEL-β3 tubulin-positive cells, and LC3-SOX10- and LC3-β3 tubulin-positive cells were determined. The white arrows indicate TUNEL-SOX10- and TUNEL-β3 tubulin-positive cells and LC3-SOX10- and LC3-β3-tubulin-positive cells, respectively. The quantitative analyses of the results are shown (c, original magnification, ×1000). Scale bars represent 10 μm (b, c). (n = 6 independent experiments in each experiments) *p < 0.05 and **p < 0.01 compared with the db/m cont group. d The expression levels of CaSR, CaMKKβ, phospho-Ser428 LKB1, phospho-Thr172 AMPK, PGC-1α, phospho-Ser1177 eNOS, Bcl-1, and β-actin of the sciatic nerve were determined. Representative western blot of CaSR, CaMKKβ, phospho-Ser428 LKB1, phospho-Thr172 AMPK, PGC-1α, phospho-Ser1177 eNOS, and β-actin and quantitative analyses of the results are shown (d). (n = 4 independent experiments in each experiments) *p < 0.05, **p < 0.01, and #p < 0.001 compared with the other groups
Discussion
The current study provides empirical evidences that cinacalcet improved sensorimotor function and restored damaged nerve phenotypes including nerve fibrosis and inflammation, axonal degeneration, loss of unmyelinated fibers, and apoptotic neuronal cell loss in the sciatic nerve of diabetic mice. Along with these changes, cinacalcet also restored defective autophagy activity in both SCs and peripheral nerve, which characterizes early-stage diabetic neuropathy. Activation of CaMKKβ and phosphorylation of LKB1 by cinacalcet increased phosphorylation of AMPK that subsequently activated PGC-1α and phospho-Ser1177 eNOS–NO and increased the ratio of Bcl-2/Bax, beclin-1, and LC3-II/LC3-I.
It is established that impaired [Ca++]i homeostasis is implicated in the development of DPN25,26. Previous studies supported the notion that deranged Ca++homeostasis is attributable to impaired sarco/endoplasmic reticulum calcium ATPase pumps located in the endoplasmic reticulum membrane19. Therapy with low-dose insulin and neurotrophin-3 restored resting Ca++ levels from intracellular stores, signifying that altered calcium homeostasis could be an early molecular marker linked to the onset of diabetic sensory neuropathy27,28. In the current study, cinacalcet treatment increased the expression of CaSR and [Ca++]i in cytoplasm in association with subsequent increase in the expression of CaMKKβ and LKB-1 in the diabetic animals and cultured SCs that was independent of adenylate energy balance, such as AMP/ATP and ADP/ATP ratios. More importantly, transfection with either CaMKKβ or LKB1 siRNA resulted in the dual suppression in the expression of both CaMKKβ and LKB1 and their downstream phospho-Thr172 AMPK, PGC-1α, and phosphor-Ser1177 eNOS signaling in HSCs cultured in high-glucose medium. The results signify the dual activation of CaMKKβ and LKB1 as a prerequisite and that the interaction between the two upstream kinases is required for further enhancement of their downstream effectors by cinacalcet treatment.
AMPK is a major downstream effector of its upstream kinases that plays a key role in cell survival and death in response to metabolic stress. The role of AMPK activation in restoring nerve function, preventing, and even reversing pathological pain associated with DPN is implicated in various studies; we previously demonstrated that fenofibrate treatment ameliorated neuronal damage in the sciatic nerve of type 2 diabetic mice by activating the PPARα-AMPK-PGC-1α-eNOS pathway15. Moreover, mitochondrial dysfunction in DPN is characterized by maladaptation in such metabolic signaling pathway as AMPK/sirtuin–PGC-1α axis that contributes to the diminishment of axonal regeneration capacity29. In line with this, diabetes-associated alterations in the peripheral nerve phenotype and concomitant development of sensorimotor dysfunction were accompanied by decreased expression of AMPK-PGC-1α-eNOS signaling and this was ameliorated by cinacalcet treatment through the upregulation of AMPK-eNOS phosphorylation in the sciatic nerve of diabetic mice.
One favorable and potential downstream mediator of AMPK signaling is NO30. Neuronal damages may be associated with secondary deficits in endothelial function resulting from impaired NO synthesis, release of NO upon endothelial injury by oxidative stress, and increased free radical activity. Cinacalcet’s favorable effects on preventing sensorimotor dysfunction and neuronal damage may be implemented by AMPK-induced modulation of eNOS and enhancement of NOx production, which preserves peripheral nerve, especially SCs, and promotes endothelial cell survival and function.
To explore the cellular fate of peripheral nerves associated with enhanced eNOS–NOx activation, we determined the degree of autophagic activity as represented by LC3-II/LC3-I. LC3-II serves as a molecular biomarker for the assessment of autophagic activity. Impaired autophagic activity participates in the development of a variety of disease including neurodegenerative disorders probably due to the accumulation of damaged molecules and organelles that promotes cell death. On the other hand, excessive autophagy could also result in cell death and dysfunction by facilitating apoptosis with potential clinical significance, and therefore, the proportional contribution of autophagy31 and apoptosis and the balance between the two counteracting functions is essential in promoting cell viability and further maintaining the functional and phenotypic integrity of peripheral nerves in diabetes. In this aspect, cinacalcet may exhibit its potential as a means to promote cell survival by enhancing autophagy and attenuating apoptosis.
In summary, this study strongly suggests a favorable effect of cinacalcet in DPN by enhancing the [Ca++]i-CaMKKβ-LKB1 pathway and its downstream effectors AMPK-PGC-1α-eNOS, which may prevent the sciatic nerve injury from diabetes-induced oxidative stress through not only decreased apoptosis but also increased autophagy activity (Fig. 9). Therefore, cinacalcet-induced AMPK activation underscores the modulation of autophagy and apoptosis in the sciatic nerve and may be a promising therapeutic means to deter and prevent the progression of DPN.
References
Sima, A. A., Thomas, P. K., Ishii, D. & Vinik, A. Diabetic neuropathies. Diabetologia 40(Suppl 3), B74–B77 (1997).
Greene, D. A., Stevens, M. J. & Feldman, E. L. Diabetic neuropathy: scope of the syndrome. Am. J. Med. 107, 2s–8s (1999).
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993).
Cameron, N. E., Eaton, S. E., Cotter, M. A. & Tesfaye, S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 44, 1973–1988 (2001).
van Dam, P. S. Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab. Res. Rev. 18, 176–184 (2002).
Anand, P. et al. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat. Med. 2, 703–707 (1996).
Yerra, V. G., Gundu, C., Bachewal, P. & Kumar, A. Autophagy: the missing link in diabetic neuropathy? Med. Hypotheses 86, 120–128 (2016).
Simmons, Z. & Feldman, E. L. Update on diabetic neuropathy. Curr. Opin. Neurol. 15, 595–603 (2002).
Tomlinson, D. R. & Gardiner, N. J. Glucose neurotoxicity. Nat. Rev. Neurosci. 9, 36–45 (2008).
Eckersley, L. Role of the Schwann cell in diabetic neuropathy. Int. Rev. Neurobiol. 50, 293–321 (2002).
Gonzalez, C. D. et al. The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy 7, 2–11 (2011).
Yu, Y. et al. Protective effect of hydrogen-rich medium against high glucose-induced apoptosis of Schwann cells in vitro. Mol. Med. Rep. 12, 3986–3992 (2015).
Hardie, D. G., Hawley, S. A. & Scott, J. W. AMP-activated protein kinase--development of the energy sensor concept. J. Physiol. 574, 7–15 (2006).
Chowdhury, S. K., Dobrowsky, R. T. & Fernyhough, P. Nutrient excess and altered mitochondrial proteome and function contribute to neurodegeneration in diabetes. Mitochondrion 11, 845–854 (2011).
Cho, Y. R. et al. Therapeutic effects of fenofibrate on diabetic peripheral neuropathy by improving endothelial and neural survival in db/db mice. PLoS ONE 9, e83204 (2014).
Shaw, R. J. et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl Acad. Sci. USA 101, 3329–3335 (2004).
Jensen, T. E. et al. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am. J. Physiol. Endocrinol. Metab. 292, E1308–E1317 (2007).
Brown, E. M. & MacLeod, R. J. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 81, 239–297 (2001).
Bukoski, R. D., Bian, K., Wang, Y. & Mupanomunda, M. Perivascular sensory nerve Ca2+ receptor and Ca2+-induced relaxation of isolated arteries. Hypertension 30, 1431–1439 (1997).
Beirowski, B. et al. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat. Neurosci. 17, 1351–1361 (2014).
Verkhratsky, A. & Fernyhough, P. Calcium signalling in sensory neurones and peripheral glia in the context of diabetic neuropathies. Cell Calcium 56, 362–371 (2014).
Dasgupta, B. & Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl Acad. Sci. USA 104, 7217–7222 (2007).
Sharma, S. S., Kumar, A., Arora, M. & Kaundal, R. K. Neuroprotective potential of combination of resveratrol and 4-amino 1,8 naphthalimide in experimental diabetic neuropathy: focus on functional, sensorimotor and biochemical changes. Free Radic. Res. 43, 400–408 (2009).
Rushton, W. A. A theory of the effects of fibre size in medullated nerve. J. Physiol. 115, 101–122 (1951).
Khomula, E. V. et al. Specific functioning of Cav3.2 T-type calcium and TRPV1 channels under different types of STZ-diabetic neuropathy. Biochim. Biophys. Acta 1832, 636–649 (2013).
Zherebitskaya, E. et al. Sensory neurons derived from diabetic rats have diminished internal Ca2+stores linked to impaired re-uptake by the endoplasmic reticulum. ASN Neuro 4, https://doi.org/10.1042/an20110038 (2012).
Burdakov, D., Petersen, O. H. & Verkhratsky, A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium 38, 303–310 (2005).
Huang, T. J., Sayers, N. M., Fernyhough, P. & Verkhratsky, A. Diabetes-induced alterations in calcium homeostasis in sensory neurones of streptozotocin-diabetic rats are restricted to lumbar ganglia and are prevented by neurotrophin-3. Diabetologia 45, 560–570 (2002).
Fernyhough, P. Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr. Diab. Rep. 15, 89 (2015).
Li, J. et al. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am. J. Physiol. Endocrinol. Metab. 287, E834–E841 (2004).
Kotiadis, V. N., Duchen, M. R. & Osellame, L. D. Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. Biochim. Biophys. Acta 1840, 1254–1265 (2014).
Acknowledgements
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (H.W.K.: NRF-2016R1A2B4015878; J.H.L.: NRF-2018R1D1A1B07048315, C.W.P.: NRF-2016R1A2B2015980), and the Seoul St. Mary’s Hospital R&D Project, The Catholic University of Korea (C.W.P.: 52015B000100004). This study was supported by the Young Investigator Research Grant from the Korean Society of Nephrology (J.H.L.; GAMBRO 2014). The authors wish to acknowledge the financial support of the St. Vincent’s Hospital, Research Institute of Medical Science (H.W.K.: SVHR-2014-04) and the Catholic Medical Center Research Foundation made in the program year of 2012.
Author information
Authors and Affiliations
Contributions
Y.C.C. and J.H.L. wrote the manuscript and researched data. H.M.O., H.W.K., M.Y.K., E.N.K. and Y.K. performed the study, analyzed data, and contributed to the discussion. Y.S.C. contributed to discussion and reviewed. H.W.K. and C.W.P. researched data, contributed to discussion, and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by G.M. Fimia
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, Y.C., Lim, J.H., Oh, H.M. et al. Calcimimetic restores diabetic peripheral neuropathy by ameliorating apoptosis and improving autophagy. Cell Death Dis 9, 1163 (2018). https://doi.org/10.1038/s41419-018-1192-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-018-1192-7
This article is cited by
-
Compound Qiying Granules alleviates diabetic peripheral neuropathy by inhibiting endoplasmic reticulum stress and apoptosis
Molecular Medicine (2023)
-
Alpha-lipoic acid activates AMPK to protect against oxidative stress and apoptosis in rats with diabetic peripheral neuropathy
Hormones (2023)
-
TXNIP, a novel key factor to cause Schwann cell dysfunction in diabetic peripheral neuropathy, under the regulation of PI3K/Akt pathway inhibition-induced DNMT1 and DNMT3a overexpression
Cell Death & Disease (2021)
-
PIAS1 alleviates diabetic peripheral neuropathy through SUMOlation of PPAR-γ and miR-124-induced downregulation of EZH2/STAT3
Cell Death Discovery (2021)
-
Sonodynamic therapy inhibits palmitate-induced beta cell dysfunction via PINK1/Parkin-dependent mitophagy
Cell Death & Disease (2019)