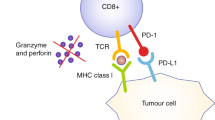
Abstract
T cell-mediated immune therapies have emerged as a promising treatment modality in different malignancies including colorectal cancer (CRC). However, only a fraction of patients currently respond to treatment. Understanding the lack of responses and finding biomarkers with predictive value is of great importance. There is evidence that CRC is a heterogeneous disease and several classification systems have been proposed that are based on genomic instability, immune cell infiltration, stromal content and molecular subtypes of gene expression. Human leukocyte antigen class I (HLA-I) plays a pivotal role in presenting processed antigens to T lymphocytes, including tumour antigens. These molecules are frequently lost in different types of cancers, including CRC, resulting in tumour immune escape from cytotoxic T lymphocytes during the natural history of cancer development. The aim of this review is to (i) summarize the prevalence and molecular mechanisms behind HLA-I loss in CRC, (ii) discuss HLA-I expression/loss in the context of the newly identified CRC molecular subtypes, (iii) analyze the HLA-I phenotypes of CRC metastases disseminated via blood or the lymphatic system, (iv) discuss strategies to recover/circumvent HLA-I expression/loss and finally (v) review the role of HLA class II (HLA-II) in CRC prognosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Garrido, F. et al. Natural history of HLA expression during tumour development. Immunol. Today 14, 491–499 (1993).
Bodmer, W. F. et al. Tumor escape from immune response by variation in HLA expression and other mechanisms. Ann. N. Y. Acad. Sci. 690, 42–49 (1993).
Marincola, F. M., Jaffee, E. M., Hicklin, D. J. & Ferrone, S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 74, 181–273 (2000).
Seliger, B., Cabrera, T., Garrido, F. & Ferrone, S. HLA class I antigen abnormalities and immune escape by malignant cells. Semin. Cancer Biol. 12, 3–13 (2002).
Aptsiauri, N., Ruiz-Cabello, F. & Garrido, F. The transition from HLA-I positive to HLA-I negative primary tumors: the road to escape from T-cell responses. Curr. Opin. Immunol. 51, 123–132 (2018).
Mendez, R. et al. Impact of HLA class I alterations in patients undergoing T cell specific immunotherapy. Immunobiology of the human MHC. Proc. 13th Int. Histocompatibility Workshop Conf. (IHWC) 2002 2, 512–514 (2006). IHWG Press.
Thor Straten, P. & Garrido, F. Targetless T cells in cancer immunotherapy. J. Immunother. Cancer 4, 23 (2016).
Carretero, R. et al. Bacillus Calmette-Guerin immunotherapy of bladder cancer induces selection of human leukocyte antigen class I-deficient tumor cells. Int. J. Cancer 129, 839–846 (2011).
Benitez, R. et al. Mutations of the beta2-microglobulin gene result in a lack of HLA class I molecules on melanoma cells of two patients immunized with MAGE peptides. Tissue Antigens 52, 520–529 (1998).
Marchand, M. et al. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int. J. Cancer 80, 219–230 (1999).
Andersen, R. et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin. Cancer Res. 22, 3734–3745 (2016).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Garrido, F., Ruiz-Cabello, F. & Aptsiauri, N. Rejection versus escape: the tumor MHC dilemma. Cancer Immunol. Immunother. 66, 259–271 (2017).
Garrido, F. & Aptsiauri, N. Cancer immune escape: MHC expression in primary tumours versus metastases. Immunology 158, 255–266 (2019).
Sade-Feldman, M. et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 8, 1136 (2017).
Tran, E. et al. T-cell transfer therapy targeting mutant KRAS in cancer. N. Engl. J. Med. 375, 2255–2262 (2016).
Garrido, F., Cabrera, T. & Aptsiauri, N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int. J. Cancer 127, 249–256 (2010).
Lampen, M. H. & van Hall, T. Strategies to counteract MHC-I defects in tumors. Curr. Opin. Immunol. 23, 293–298 (2011).
Ugurel, S. et al. MHC class-I downregulation in PD-1/PD-L1 inhibitor refractory Merkel cell carcinoma and its potential reversal by histone deacetylase inhibition: a case series. Cancer Immunol. Immunother. 68, 983–990 (2019).
Csiba, A., Whitwell, H. L. & Moore, M. Distribution of histocompatibility and leucocyte differentiation antigens in normal human colon and in benign and malignant colonic neoplasms. Br. J. Cancer 50, 699–709 (1984).
Momburg, F. et al. Loss of HLA-A,B,C and de novo expression of HLA-D in colorectal cancer. Int. J. Cancer 37, 179–184 (1986).
Moore, M., Ghosh, A. K., Johnston, D. & Street, A. J. Expression of MHC class II products on human colorectal cancer. An immunohistological and flow cytometric study. J. Immunogenet. 13, 201–209 (1986).
Gutierrez, J. et al. Class I and II HLA antigen distribution in normal mucosa, adenoma and colon carcinoma: relation with malignancy and invasiveness. Exp. Clin. Immunogenet. 4, 144–152 (1987).
Durrant, L. G. et al. Quantitation of MHC antigen expression on colorectal tumours and its association with tumour progression. Br. J. Cancer 56, 425–432 (1987).
Rees, R. C. et al. Loss of polymorphic A and B locus HLA antigens in colon carcinoma. Br. J. Cancer 57, 374–377 (1988).
Smith, M. E., Bodmer, W. F. & Bodmer, J. G. Selective loss of HLA-A,B,C locus products in colorectal adenocarcinoma. Lancet 1, 823–824 (1988).
Lopez-Nevot, M. A. et al. HLA class I gene expression on human primary tumours and autologous metastases: demonstration of selective losses of HLA antigens on colorectal, gastric and laryngeal carcinomas. Br. J. Cancer 59, 221–226 (1989).
Vogelstein, B. et al. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 319, 525–532 (1988).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Tsioulias, G. J. et al. Expression of HLA class I antigens in sporadic adenomas and histologically normal mucosa of the colon. Cancer Res. 53, 2374–2378 (1993).
Lakatos, E. et al. Evolutionary dynamics of neoantigens in growing tumors. Nat. Genet. 52, 1057–1066 (2020).
Kloor, M. et al. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int. J. Cancer 121, 454–458 (2007).
Horie, Y., Chiba, M., Iizuka, M., Igarashi, K. & Masamune, O. HLA antigens on colorectal adenoma and cancer cells. Tohoku J. Exp. Med. 160, 311–322 (1990).
Smith, M. E., Marsh, S. G., Bodmer, J. G., Gelsthorpe, K. & Bodmer, W. F. Loss of HLA-A,B,C allele products and lymphocyte function-associated antigen 3 in colorectal neoplasia. Proc. Natl Acad. Sci. USA 86, 5557–5561 (1989).
Chen, H. et al. Genomic and immune profiling of pre-invasive lung adenocarcinoma. Nat. Commun. 10, 5472 (2019).
Moller, P., Koretz, K., Schlag, P. & Momburg, F. Frequency of abnormal expression of HLA-A,B,C and HLA-DR molecules, invariant chain, and LFA-3 (CD58) in colorectal carcinoma and its impact on tumor recurrence. Int. J. Cancer Suppl. 6, 155–162 (1991).
Cabrera, T. et al. High frequency of altered HLA class I phenotypes in invasive colorectal carcinomas. Tissue Antigens 52, 114–123 (1998).
Ruiz-Cabello, F. et al. Molecular analysis of MHC-class-I alterations in human tumor cell lines. Int. J. Cancer Suppl. 6, 123–130 (1991).
Browning, M. et al. Mechanisms of loss of HLA class I expression on colorectal tumor cells. Tissue Antigens 47, 364–371 (1996).
Kaklamanis, L. et al. Loss of HLA class-I alleles, heavy chains and beta 2-microglobulin in colorectal cancer. Int. J. Cancer 51, 379–385 (1992).
Kloor, M., Michel, S. & von Knebel Doeberitz, M. Immune evasion of microsatellite unstable colorectal cancers. Int. J. Cancer 127, 1001–1010 (2010).
Ionov, Y., Peinado, M. A., Malkhosyan, S., Shibata, D. & Perucho, M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363, 558–561 (1993).
Weisenberger, D. J. et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 38, 787–793 (2006).
Kloor, M. & von Knebel Doeberitz, M. The immune biology of microsatellite-unstable cancer. Trends Cancer 2, 121–133 (2016).
Cabrera, C. M. et al. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: beta2-microglobulin inactivation in MSI-positive tumors and LMP7/TAP2 downregulation in MSI-negative tumors. Tissue Antigens 61, 211–219 (2003).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Bernal, M. et al. Genome-wide differential genetic profiling characterizes colorectal cancers with genetic instability and specific routes to HLA class I loss and immune escape. Cancer Immunol. Immunother. 61, 803–816 (2012).
Bernal, M., Ruiz-Cabello, F., Concha, A., Paschen, A. & Garrido, F. Implication of the beta2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol. Immunother. 61, 1359–1371 (2012).
Bicknell, D. C., Kaklamanis, L., Hampson, R., Bodmer, W. F. & Karran, P. Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr. Biol. 6, 1695–1697 (1996).
Kloor, M. et al. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 65, 6418–6424 (2005).
Cabrera, C. M., Lopez-Nevot, M. A., Jimenez, P. & Garrido, F. Involvement of the chaperone tapasin in HLA-B44 allelic losses in colorectal tumors. Int. J. Cancer 113, 611–618 (2005).
Dierssen, J. W. et al. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer 7, 33 (2007).
Grasso, C. S. et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov. 8, 730–749 (2018).
Ling, A. et al. TAP1 down-regulation elicits immune escape and poor prognosis in colorectal cancer. Oncoimmunology 6, e1356143 (2017).
Yoshihama, S. et al. NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc. Natl Acad. Sci. USA 113, 5999–6004 (2016).
Gurjao, C. et al. Intrinsic resistance to immune checkpoint blockade in a mismatch repair-deficient colorectal cancer. Cancer Immunol. Res. 7, 1230–1236 (2019).
Umar, A., Risinger, J. I., Hawk, E. T. & Barrett, J. C. Testing guidelines for hereditary non-polyposis colorectal cancer. Nat. Rev. Cancer 4, 153–158 (2004).
Clendenning, M. et al. Somatic mutations of the coding microsatellites within the beta-2-microglobulin gene in mismatch repair-deficient colorectal cancers and adenomas. Fam. Cancer 17, 91–100 (2018).
de Miranda, N. F. et al. Infiltration of Lynch colorectal cancers by activated immune cells associates with early staging of the primary tumor and absence of lymph node metastases. Clin. Cancer Res. 18, 1237–1245 (2012).
Becht, E. et al. Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin. Cancer Res. 22, 4057–4066 (2016).
Karpinski, P., Rossowska, J. & Sasiadek, M. M. Immunological landscape of consensus clusters in colorectal cancer. Oncotarget 8, 105299–105311 (2017).
Soldevilla, B. et al. The correlation between immune subtypes and consensus molecular subtypes in colorectal cancer identifies novel tumour microenvironment profiles, with prognostic and therapeutic implications. Eur. J. Cancer 123, 118–129 (2019).
Ijsselsteijn, M. E. et al. Revisiting immune escape in colorectal cancer in the era of immunotherapy. Br. J. Cancer 120, 815–818 (2019).
Pino, M. S. & Chung, D. C. The chromosomal instability pathway in colon cancer. Gastroenterology 138, 2059–2072 (2010).
Maleno, I. et al. Distribution of HLA class I altered phenotypes in colorectal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Immunogenetics 56, 244–253 (2004).
Maleno, I. et al. Frequent loss of heterozygosity in the beta2-microglobulin region of chromosome 15 in primary human tumors. Immunogenetics 63, 65–71 (2011).
Jimenez, P. et al. Chromosome loss is the most frequent mechanism contributing to HLA haplotype loss in human tumors. Int. J. Cancer 83, 91–97 (1999).
van den Bulk, J. et al. Neoantigen-specific immunity in low mutation burden colorectal cancers of the consensus molecular subtype 4. Genome Med. 11, 87 (2020).
Luke, J. J., Bao, R., Sweis, R. F., Spranger, S. & Gajewski, T. F. WNT/beta-catenin pathway activation correlates with immune exclusion across human cancers. Clin. Cancer Res. 25, 3074–3083 (2019).
Xue, J. et al. Intrinsic beta-catenin signaling suppresses CD8(+) T-cell infiltration in colorectal cancer. Biomed. Pharmacother. 115, 108921 (2019).
Versteeg, R., Noordermeer, I. A., Kruse-Wolters, M., Ruiter, D. J. & Schrier, P. I. c-myc down-regulates class I HLA expression in human melanomas. EMBO J. 7, 1023–1029 (1988).
Yang, W., Li, Y., Gao, R., Xiu, Z. & Sun, T. MHC class I dysfunction of glioma stem cells escapes from CTL-mediated immune response via activation of Wnt/beta-catenin signaling pathway. Oncogene 39, 1098–1111 (2019).
Young, A., Lou, D. & McCormick, F. Oncogenic and wild-type Ras play divergent roles in the regulation of mitogen-activated protein kinase signaling. Cancer Discov. 3, 112–123 (2013).
Atkins, D. et al. MHC class I antigen processing pathway defects, ras mutations and disease stage in colorectal carcinoma. Int. J. Cancer 109, 265–273 (2004).
Ledys, F. et al. RAS status and neoadjuvant chemotherapy impact CD8+ cells and tumor HLA class I expression in liver metastatic colorectal cancer. J. Immunother. Cancer 6, 123 (2018).
Klampfer, L. et al. Oncogenic Ki-ras inhibits the expression of interferon-responsive genes through inhibition of STAT1 and STAT2 expression. J. Biol. Chem. 278, 46278–46287 (2003).
Liao, W. et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell. 35, 559–72 e7 (2019).
Sers, C. et al. Down-regulation of HLA Class I and NKG2D ligands through a concerted action of MAPK and DNA methyltransferases in colorectal cancer cells. Int. J. Cancer 125, 1626–1639 (2009).
Coebergh van den Braak, R. R. J. et al. Interconnectivity between molecular subtypes and tumor stage in colorectal cancer. BMC Cancer 20, 850 (2020).
Yoo, S. Y. et al. Whole-slide image analysis reveals quantitative landscape of tumor-immune microenvironment in colorectal cancers. Clin. Cancer Res. 26, 870–881 (2020).
Garrido, F. et al. The escape of cancer from T cell-mediated immune surveillance: HLA Class I loss and tumor tissue architecture. Vaccines (Basel). 5, 7 (2017).
Perea, F. et al. HLA class I loss and PD-L1 expression in lung cancer: impact on T-cell infiltration and immune escape. Oncotarget 9, 4120–4133 (2017).
Geiser, A. G. et al. Transforming growth factor beta 1 (TGF-beta 1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-beta 1 null mouse phenotype. Proc. Natl Acad. Sci. USA 90, 9944–9948 (1993).
Ma, D. & Niederkorn, J. Y. Transforming growth factor-beta down-regulates major histocompatibility complex class I antigen expression and increases the susceptibility of uveal melanoma cells to natural killer cell-mediated cytolysis. Immunology 86, 263–269 (1995).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–30.e14 (2018).
Romero, I. et al. MHC intratumoral heterogeneity may predict cancer progression and response to immunotherapy. Front. Immunol. 9, 102 (2018).
del Campo, A. B. et al. Immune escape of cancer cells with beta2-microglobulin loss over the course of metastatic melanoma. Int. J. Cancer 134, 102–113 (2014).
Carretero, R. et al. Analysis of HLA class I expression in progressing and regressing metastatic melanoma lesions after immunotherapy. Immunogenetics 60, 439–447 (2008).
Menon, A. G. et al. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab. Invest. 84, 493–501 (2004).
Garrido, F. et al. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol. Today 18, 89–95 (1997).
Watson, N. F. et al. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int. J. Cancer 118, 6–10 (2006).
Demanet, C. et al. Down-regulation of HLA-A and HLA-Bw6, but not HLA-Bw4, allospecificities in leukemic cells: an escape mechanism from CTL and NK attack? Blood 103, 3122–3130 (2004).
Cabrera, T., Lopez-Nevot, M. A., Gaforio, J. J., Ruiz-Cabello, F. & Garrido, F. Analysis of HLA expression in human tumor tissues. Cancer Immunol. Immunother. 52, 1–9 (2003).
Rodriguez, T. et al. Distinct mechanisms of loss of IFN-gamma mediated HLA class I inducibility in two melanoma cell lines. BMC Cancer 7, 34 (2007).
del Campo, A. B. et al. Efficient recovery of HLA class I expression in human tumor cells after beta2-microglobulin gene transfer using adenoviral vector: implications for cancer immunotherapy. Scand. J. Immunol. 70, 125–135 (2009).
del Campo, A. B. et al. Adenovirus expressing beta2-microglobulin recovers HLA class I expression and antitumor immunity by increasing T-cell recognition. Cancer Gene Ther. 21, 317–332 (2014).
del Campo, A. B., Carretero, J., Aptsiauri, N. & Garrido, F. Targeting HLA class I expression to increase tumor immunogenicity. Tissue Antigens 79, 147–154 (2012).
Pulido, M. et al. Restoration of MHC-I on tumor cells by Fhit transfection promotes immune rejection and acts as an individualized immunotherapeutic vaccine. Cancers (Basel). 12, 1563 (2020).
Rodriguez, G. M. et al. NLRC5 elicits antitumor immunity by enhancing processing and presentation of tumor antigens to CD8(+) T lymphocytes. Oncoimmunology 5, e1151593 (2016).
Kalbasi, A. et al. Uncoupling interferon signaling and antigen presentation to overcome immunotherapy resistance due to JAK1 loss in melanoma. Sci. Transl. Med. 12, eabb0152 (2020).
Marincola, F. M. et al. Locus-specific analysis of human leukocyte antigen class I expression in melanoma cell lines. J. Immunother. Emphas. Tumor Immunol. 16, 13–23 (1994).
Respa, A. et al. Association of IFN-gamma signal transduction defects with impaired HLA class I antigen processing in melanoma cell lines. Clin. Cancer Res. 17, 2668–2678 (2011).
Sucker, A. et al. Acquired IFNgamma resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat. Commun. 8, 15440 (2017).
Simpson, J. A. et al. Intratumoral T cell infiltration, MHC class I and STAT1 as biomarkers of good prognosis in colorectal cancer. Gut 59, 926–933 (2010).
Propper, D. J. et al. Low-dose IFN-gamma induces tumor MHC expression in metastatic malignant melanoma. Clin. Cancer Res. 9, 84–92 (2003).
Zhang, S. et al. Systemic interferon-gamma increases MHC class I expression and T-cell infiltration in cold tumors: results of a phase 0 clinical trial. Cancer Immunol. Res. 7, 1237–1243 (2019).
Serrano, A. et al. Rexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2’-deoxycytidine treatment. Int. J. Cancer 94, 243–251 (2001).
Park, J., Thomas, S. & Munster, P. N. Epigenetic modulation with histone deacetylase inhibitors in combination with immunotherapy. Epigenomics 7, 641–652 (2015).
Vlkova, V. et al. Epigenetic regulations in the IFNgamma signalling pathway: IFNgamma-mediated MHC class I upregulation on tumour cells is associated with DNA demethylation of antigen-presenting machinery genes. Oncotarget 5, 6923–6935 (2014).
Li, H. et al. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget 5, 587–598 (2014).
Luo, N. et al. DNA methyltransferase inhibition upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat. Commun. 9, 248 (2018).
Wang, X. et al. Histone deacetylase inhibition sensitizes PD1 blockade-resistant B-cell lymphomas. Cancer Immunol. Res. 7, 1318–1331 (2019).
Ebert, P. J. R. et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity 44, 609–621 (2016).
Kang, S. H. et al. Inhibition of MEK with trametinib enhances the efficacy of anti-PD-L1 inhibitor by regulating anti-tumor immunity in head and neck squamous cell carcinoma. Oncoimmunology 8, e1515057 (2019).
Liu, L. et al. The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin. Cancer Res. 21, 1639–1651 (2015).
Huyghe, N., Baldin, P. & Van den Eynde, M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: what is the future beyond deficient mismatch-repair tumours? Gastroenterol. Rep. (Oxf.). 8, 11–24 (2019).
Garrido, G. et al. Upregulation of HLA class I expression on tumor cells by the anti-EGFR antibody nimotuzumab. Front. Pharmacol. 8, 595 (2017).
Angell, T. E., Lechner, M. G., Jang, J. K., LoPresti, J. S. & Epstein, A. L. MHC class I loss is a frequent mechanism of immune escape in papillary thyroid cancer that is reversed by interferon and selumetinib treatment in vitro. Clin. Cancer Res. 20, 6034–6044 (2014).
Lazzari, C. et al. Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: is this the beginning of the end for cancer? Ther. Adv. Med. Oncol. 10, 1758835918762094 (2018).
Garnett, C. T. et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 64, 7985–7994 (2004).
Garrido, C. et al. Immunotherapy eradicates metastases with reversible defects in MHC class I expression. Cancer Immunol. Immunother. 60, 1257–1268 (2011).
Reits, E. A. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203, 1259–1271 (2006).
Garrido, F., Aptsiauri, N., Doorduijn, E. M., Garcia Lora, A. M. & van Hall, T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 39, 44–51 (2016).
Ardolino, M. et al. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J. Clin. Invest. 124, 4781–4794 (2014).
Seo, H. et al. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat. Commun. 8, 15776 (2017).
van Hall, T. et al. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat. Med. 12, 417–424 (2006).
Wolpert, E. Z. et al. Generation of CD8+ T cells specific for transporter associated with antigen processing deficient cells. Proc. Natl Acad. Sci. USA 94, 11496–11501 (1997).
Wang, X. et al. MHC class I-independent activation of virtual memory CD8 T cells induced by chemotherapeutic agent-treated cancer cells. Cell. Mol. Immunol. https://doi.org/10.1038/s41423-020-0463-2 (2020).
Concha, A. et al. Different patterns of HLA-DR antigen expression in normal epithelium, hyperplastic and neoplastic malignant lesions of the breast. Eur. J. Immunogenet. 22, 299–310 (1995).
Bedossa, P. et al. Expression of histocompatibility antigens and characterization of the lymphocyte infiltrate in hyperplastic polyps of the large bowel. Hum. Pathol. 21, 319–324 (1990).
Lovig, T. et al. Strong HLA-DR expression in microsatellite stable carcinomas of the large bowel is associated with good prognosis. Br. J. Cancer 87, 756–762 (2002).
Seliger, B., Ruiz-Cabello, F. & Garrido, F. IFN inducibility of major histocompatibility antigens in tumors. Adv. Cancer Res. 101, 249–276 (2008).
Axelrod, M. L., Cook, R. S., Johnson, D. B. & Balko, J. M. Biological consequences of MHC-II expression by tumor cells in cancer. Clin. Cancer Res. 25, 2392–2402 (2019).
Cabrera, T., Ruiz-Cabello, F. & Garrido, F. Biological implications of HLA-DR expression in tumours. Scand. J. Immunol. 41, 398–406 (1995).
Seliger, B., Kloor, M. & Ferrone, S. HLA class II antigen-processing pathway in tumors: Molecular defects and clinical relevance. Oncoimmunology 6, e1171447 (2017).
Dunne, M. R. et al. Characterising the prognostic potential of HLA-DR during colorectal cancer development. Cancer Immunol. Immunother. 69, 1577–1588 (2020).
Walsh, M. D. et al. HLA-DR expression is associated with better prognosis in sporadic Australian clinicopathological Stage C colorectal cancers. Int. J. Cancer 125, 1231–1237 (2009).
Michel, S. et al. Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int. J. Cancer 127, 889–898 (2010).
Surmann, E. M. et al. Association of high CD4-positive T cell infiltration with mutations in HLA class II-regulatory genes in microsatellite-unstable colorectal cancer. Cancer Immunol. Immunother. 64, 357–366 (2015).
Warabi, M., Kitagawa, M. & Hirokawa, K. Loss of MHC class II expression is associated with a decrease of tumor-infiltrating T cells and an increase of metastatic potential of colorectal cancer: immunohistological and histopathological analyses as compared with normal colonic mucosa and adenomas. Pathol. Res. Pract. 196, 807–815 (2000).
Dienstmann, R. et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 17, 79–92 (2017).
Waldburger, J. M. et al. Lessons from the bare lymphocyte syndrome: molecular mechanisms regulating MHC class II expression. Immunol. Rev. 178, 148–165 (2000).
Garrido, F. MHC/HLA class I loss in cancer cells. Adv. Exp. Med Biol. 1151, 1–95, https://doi.org/10.1007/978-3-030-17864-2 (2019). ISBN 978-3-030-17864-2 (eBook).
Acknowledgements
Biological samples and associated data of the patients included in the study were collected, processed and provided by the Biobank for Biomedical Research and Public Health of the Valencian Community (Biobank IBSP-CV) authorized in the National Registry of Biobanks Carlos III Health Institute (code number B.0000863), which is part of the Biobank Network of Valencia and the National Biobank Network Platform (PT13/0010/0064), following standard operating procedures, and their activity is supported by Ethics Committees and Scientific Committees. This work was supported by the grants from Instituto de Salud Carlos III, co-financed by European Regional Development Fund (FEDER) [PI11/01386, PI14/01978, PI16/00752, PI17/00197 and PI18/00826]. P. A. is supported by the Consejería de Salud, Junta de Andalucía through the contract ‘Nicolás Monardes’ [C-0013-2018]. We would like to thank Dr. Mónica Bernal for her excellent help in designing and making the figures included in this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Anderson, P., Aptsiauri, N., Ruiz-Cabello, F. et al. HLA class I loss in colorectal cancer: implications for immune escape and immunotherapy. Cell Mol Immunol 18, 556–565 (2021). https://doi.org/10.1038/s41423-021-00634-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-021-00634-7