Abstract
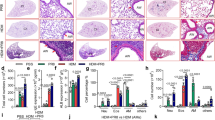
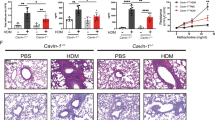
Interleukin 5 (IL-5) plays crucial roles in type 2-high asthma by mediating eosinophil maturation, activation, chemotaxis and survival. Inhibition of IL-5 signaling is considered a strategy for asthma treatment. Here, we identified MARCH2 and MARCH3 as critical negative regulators of IL-5-triggered signaling. MARCH2 and MARCH3 associate with the IL-5 receptor α chain (IL-5Rα) and mediate its K27-linked polyubiquitination at K379 and K383, respectively, and its subsequent lysosomal degradation. Deficiency of MARCH2 or MARCH3 modestly increases the level of IL-5Rα and enhances IL-5-induced signaling, whereas double knockout of MARCH2/3 has a more dramatic effect. March2/3 double knockout markedly increases the proportions of eosinophils in the bone marrow and peripheral blood in mice. Double knockout of March2/3 aggravates ovalbumin (OVA)-induced eosinophilia and causes increased inflammatory cell infiltration, peribronchial mucus secretion and production of Th2 cytokines. Neutralization of Il-5 attenuates OVA-induced airway inflammation and the enhanced effects of March2/3 double deficiency. These findings suggest that MARCH2 and MARCH3 play redundant roles in targeting IL-5Rα for degradation and negatively regulating allergic airway inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are presented in the paper. The materials described in the study are either commercially available or available upon request from the corresponding author.
References
Yanagibashi T, Satoh M, Nagai Y, Koike M, Takatsu K. Allergic diseases: From bench to clinic - contribution of the discovery of interleukin-5. Cytokine. 2017;98:59–70.
Dougan M, Dranoff G, Dougan SK. GM-CSF, IL-3, and IL-5 family of cytokines: regulators of inflammation. Immunity. 2019;50:796–811.
Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7.
Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184:1469–85.
FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–41.
Rothenberg ME. Humanized anti-IL-5 antibody therapy. Cell. 2016;165:509.
Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–66.
Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–9.
Tomaki M, Zhao LL, Lundahl J, Sjostrand M, Jordana M, Linden A, et al. Eosinophilopoiesis in a murine model of allergic airway eosinophilia: involvement of bone marrow IL-5 and IL-5 receptor alpha. J Immunol. 2000;165:4040–50.
Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, et al. The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev. 2012;250:277–302.
Ogata N, Kouro T, Yamada A, Koike M, Hanai N, Ishikawa T, et al. JAK2 and JAK1 constitutively associate with an interleukin-5 (IL-5) receptor alpha and betac subunit, respectively, and are activated upon IL-5 stimulation. Blood. 1998;91:2264–71.
Takaki S, Kanazawa H, Shiiba M, Takatsu K. A critical cytoplasmic domain of the interleukin-5 (IL-5) receptor alpha chain and its function in IL-5-mediated growth signal transduction. Mol Cell Biol. 1994;14:7404–13.
Stout BA, Bates ME, Liu LY, Farrington NN, Bertics PJ. IL-5 and granulocyte-macrophage colony-stimulating factor activate STAT3 and STAT5 and promote Pim-1 and cyclin D3 protein expression in human eosinophils. J Immunol. 2004;173:6409–17.
Yun Y, Kanda A, Kobayashi Y, Van Bui D, Suzuki K, Sawada S, et al. Increased CD69 expression on activated eosinophils in eosinophilic chronic rhinosinusitis correlates with clinical findings. Allergol Int. 2020;69:232–8.
Sonkin D, Palmer M, Rong X, Horrigan K, Regnier CH, Fanton C, et al. The identification and characterization of a STAT5 gene signature in hematologic malignancies. Cancer Biomark. 2015;15:79–87.
Kagami S, Nakajima H, Kumano K, Suzuki K, Suto A, Imada K, et al. Both stat5a and stat5b are required for antigen-induced eosinophil and T-cell recruitment into the tissue. Blood. 2000;95:1370–7.
Zhu Y, Chen L, Huang Z, Alkan S, Bunting KD, Wen R, et al. Cutting edge: IL-5 primes Th2 cytokine-producing capacity in eosinophils through a STAT5-dependent mechanism. J Immunol. 2004;173:2918–22.
Samji T, Hong S, Means RE. The Membrane Associated RING-CH Proteins: A Family of E3 Ligases with Diverse Roles through the Cell. Int Sch Res Not. 2014;2014:637295.
Lin H, Li S, Shu HB. The Membrane-Associated MARCH E3 Ligase Family: Emerging Roles in Immune Regulation. Front Immunol. 2019;10:1751.
Zheng C. The emerging roles of the MARCH ligases in antiviral innate immunity. Int J Biol Macromol. 2021;171:423–7.
Chen R, Li M, Zhang Y, Zhou Q, Shu HB. The E3 ubiquitin ligase MARCH8 negatively regulates IL-1beta-induced NF-kappaB activation by targeting the IL1RAP coreceptor for ubiquitination and degradation. Proc Natl Acad Sci USA 2012;109:14128–33.
Lin H, Gao D, Hu MM, Zhang M, Wu XX, Feng L, et al. MARCH3 attenuates IL-1beta-triggered inflammation by mediating K48-linked polyubiquitination and degradation of IL-1RI. Proc Natl Acad Sci USA 2018;115:12483–8.
Lin H, Feng L, Cui KS, Zeng LW, Gao D, Zhang LX, et al. The membrane-associated E3 ubiquitin ligase MARCH3 downregulates the IL-6 receptor and suppresses colitis-associated carcinogenesis. Cell Mol Immunol. 2021;18:2648–59.
Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–4.
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–7.
Nakamura N. The role of the transmembrane RING finger proteins in cellular and organelle function. Membr (Basel). 2011;1:354–93.
Chathuranga K, Kim TH, Lee H, Park JS, Kim JH, Chathuranga WAG, et al. Negative regulation of NEMO signaling by the ubiquitin E3 ligase MARCH2. EMBO J. 2020;39:e105139.
Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–7.
Iwasaki H, Mizuno S, Mayfield R, Shigematsu H, Arinobu Y, Seed B, et al. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J Exp Med. 2005;201:1891–7.
Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74.
Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: Eosinophilic airway inflammation in nonallergic asthma. Nat Med. 2013;19:977–9.
Lambrecht BN, Hammad H, Fahy JV. The Cytokines of Asthma. Immunity. 2019;50:975–91.
Kitamura T, Tange T, Terasawa T, Chiba S, Kuwaki T, Miyagawa K, et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989;140:323–34.
Fu Z, Yu C, Wang L, Gao K, Xu G, Wang W, et al. Development of a robust reporter gene based assay for the bioactivity determination of IL-5-targeted therapeutic antibodies. J Pharm Biomed Anal. 2018;148:280–7.
Casaro M, Souza VR, Oliveira FA, Ferreira CM. OVA-induced allergic airway inflammation mouse model. Methods Mol Biol. 2019;1916:297–301.
Zhang HX, Xu ZS, Lin H, Li M, Xia T, Cui K, et al. TRIM27 mediates STAT3 activation at retromer-positive structures to promote colitis and colitis-associated carcinogenesis. Nat Commun. 2018;9:3441.
Tong J, Bandulwala HS, Clay BS, Anders RA, Shilling RA, Balachandran DD, et al. Fas-positive T cells regulate the resolution of airway inflammation in a murine model of asthma. J Exp Med. 2006;203:1173–84.
Acknowledgements
We thank Deng Gao, Xuemei Yi, Mi Li, Ru Zang, Chen Li, and Li Zhong for technical help and academic discussions and sincerely appreciate all the staff at the core facility of the Medical Research Institute at Wuhan University for their technical support. This work was supported by grants from the National Natural Science Foundation of China (32188101, 31830024 and 32070775), the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-071) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
SL, H-BS, and L-WZ conceived and designed the study. L-WZ, LF, RL, and HL performed the experiments. SL, L-WZ, and H-BS analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, LW., Feng, L., Liu, R. et al. The membrane-associated ubiquitin ligases MARCH2 and MARCH3 target IL-5 receptor alpha to negatively regulate eosinophilic airway inflammation. Cell Mol Immunol 19, 1117–1129 (2022). https://doi.org/10.1038/s41423-022-00907-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-022-00907-9
Keywords
This article is cited by
-
RNF173 suppresses RAF/MEK/ERK signaling to regulate invasion and metastasis via GRB2 ubiquitination in Hepatocellular Carcinoma
Cell Communication and Signaling (2023)