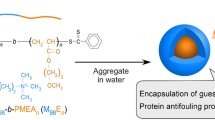
Abstract
The natural bioactive compound caffeic acid phenethyl ester (CAPE) possesses antioxidant, antiinflammatory and anticancer activity. However, the in vivo application of CAPE is limited due to its poor solubility in aqueous media. In this contribution, we report a strategy for enhancing the solubility of CAPE in water by novel micellar carriers comprising segments structurally similar to the CAPE molecule. A series of amphiphilic poly(ethylene oxide)-b-poly(α-cinnamyl-ε-caprolactone-co-ε-caprolactone)-b-poly(ethylene oxide) (PEO-b-P(CyCL-co-CL)-b-PEO) triblock copolymers were synthesized by combining ring-opening copolymerization and “click” reactions. Calculations of the Flory–Huggins parameter suggested that P(CyCL-co-CL) copolymers have a higher affinity for CAPE than do PCL polymer. Micellar carriers based on PEO-b-P(CyCL-co-CL)-b-PEO were formed via the solvent evaporation method and then loaded with CAPE. Dynamic light scattering (DLS) and cryogenic transmission electron microscopy (cryo-TEM) revealed the formation of nanosized spherical micelles that maintained their structural integrity upon dilution to 0.055–0.06 g L−1. The main characteristics of the PEO-b-P(CyCL-co-CL)-b-PEO systems were compared to those of the PEO-b-PCL-b-PEO system. The attachment of pendant cinnamyl moieties to the hydrophobic PCL block enhanced the encapsulation efficiency of the micelles and reduced their burst release behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118:6844–92.
Deshmukh AS, Chauhan PN, Noolvi MN, Chaturvedi K, Ganguly K, Shukla SS, et al. Polymeric micelles: Basic research to clinical practice. Int J Pharm. 2017;532:249–68.
Gong J, Chen M, Zheng Y, Wang S, Wang Y. Polymeric micelles drug delivery system in oncology. J Control Release. 2012;159:312–23.
Cabral H, Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Control Release. 2014;190:465–76.
Danson S, Ferry D, Alakhov V, Margison J, Kerr D, Jowle D, et al. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br J Cancer. 2004;90:2085–91.
Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–63.
Miyata K, Kakizawa Y, Nishiyama N, Yamasaki Y, Watanabe T, Kohara M, et al. Freeze-dried formulations for in vivo gene delivery of PEGylated polyplex micelles with disulfide crosslinked cores to the liver. J Control Release. 2005;109:15–23.
Lavasanifar A, Samuel J, Sattari S, Kwon GS. Block copolymer micelles for the encapsulation and delivery of Amphotericin B. Pharm Res. 2002;19:418–22.
Falamarzian A, Lavasanifar A. Chemical modification of hydrophobic block in poly(ethylene oxide) poly(caprolactone) based nanocarriers: Еffect on the solubilization and hemolytic activity of Amphotericin B. Macromol Biosci. 2010;10:648–56.
Falamarzian A, Lavasanifar A. Optimization of the hydrophobic domain in poly(ethylene oxide)-poly(-caprolactone) based nano-carriers for the solubilization and delivery of Amphotericin B. Colloids Surf B: Biointerfaces. 2010;81:313–20.
Mahmud A, Patel S, Molavi O, Choi P, Samuel J, Lavasanifar A. Self-associating poly(ethylene oxide)-b-poly(α-cholesteryl carboxylate-ε-caprolactone) block copolymer for the solubilization of stat-3 inhibitor Cucurbitacin I. Biomacromolecules. 2009;10:471–8.
Chen Y-J, Shiao MS, Wang SY. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anti-Cancer Drugs. 2001;12:143–9.
Orban Z, Mitsiades N, Burke TR Jr, Tsokos M, Chrousos GP. Caffeic acid phenethyl ester induces leukocyte apoptosis, modulates nuclear factor-kappa B and suppresses acute inflammation. Neuroimmunomodulation. 2000;7:99–105.
Akyol S, Ozturk G, Ginis Z, Armutcu F, Yigitoglu MR, Akyol O. In vivo and in vitro antineoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives. Nutr Cancer. 2013;65:515–26.
Tsai TH, Yu CH, Chang YP, Lin YT, Huang CJ, Kuo YH, et al. Protective effect of caffeic acid derivatives on tert-butyl hydroperoxideinduced oxidative hepato-toxicity and mitochondrial dysfunction in HepG2 cells. Molecules. 2017;22:702.
Derman S. Caffeic acid phenethyl ester loaded PLGA nanoparticles: effect of various process parameters on reaction yield, encapsulation efficiency, and particle size. J Nanomater. 2015; 341848. https://www.hindawi.com/journals/jnm/2015/341848/cta/
Lee HY, Jeong YI, Kim EJ, Lee KD, Choi SH, Kim YJ, et al. Preparation of caffeic acid phenethyl ester-incorporated nanoparticles and their biological activity. J Pharm Sci. 2015;104:144–54.
Yoncheva K, Tzankova V, Yordanov Y, Tzankov B, Grancharov G, Aluani D, et al. Evaluation of antioxidant activity of caffeic acid phenethyl ester loaded block copolymer micelles. Biotechnol Biotechnol Equip. 2019;33:64–74.
Darcos V, El Habnouni S, Nottelet B, El Ghzaoui A, Coudane J. Well-defined PCL-graft-PDMAEMA prepared by ring-opening polymerisation and click chemistry. Polym Chem. 2010;1:280–2.
Flory PJ. Principle of polymer chemistry. Ithaca, NY: Cornell University Press; 1953.
Fedors RF. A method for estimating both the solubility parameters and molar volumes of liquids. J Polym Eng Sci. 1974;14:147–54.
Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed. 2001;40:2004–21.
Parrish B, Breitenkamp R, Emrick T. PEG- and peptide-grafted aliphatic polyesters by click chemistry. J Am Chem Soc. 2005;127:7404–10.
Quaglia F, Ostacolo L, De Rosa G, La Rotonda MI, Ammendola M, Nese G, et al. Nanoscopic core-shell drug carriers made of amphiphilic triblock and star-diblock copolymers. Int J Pharm. 2006;324:56–66.
Samad A, Bakkour Y, Fanny C, Omar F, Coudane J, Nottelet B. From nanospheres to micelles: simple control of PCL-g-PEG copolymers amphiphilicity through thiol-yne photografting. Polym Chem. 2015;6:5093–102.
ISO 10993-5:2009. Biological evaluation of medical devices–Part 5: Tests for in vitro cytotoxicity. International Organization for Standardization. 2009. https://www.iso.org/standard/36406.html. Accessed 26 Sept 2019.
Acknowledgements
This work was supported by the Bulgarian National Science Fund [Grant number DN 09-1/2016]. The authors thank Mrs R. Radeva and Dr Ch. Novakov for GPC measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Grancharov, G., Atanasova, MD., Aluani, D. et al. Functional block copolymers bearing pendant cinnamyl groups for enhanced solubilization of caffeic acid phenethyl ester. Polym J 52, 435–447 (2020). https://doi.org/10.1038/s41428-019-0297-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-019-0297-x
This article is cited by
-
In vitro evaluation of antioxidant activity and biocompatibility of caffeic acid phenethyl ester loaded in polymeric micelles
Molecular & Cellular Toxicology (2023)
-
Optimization of extraction process parameters of caffeic acid from microalgae by supercritical carbon dioxide green technology
BMC Chemistry (2022)
-
Enzyme-catalyzed propagation of cello-oligosaccharide chains from bifunctional oligomeric primers for the preparation of block co-oligomers and their crystalline assemblies
Polymer Journal (2021)
-
Mixed micellar system for codelivery of doxorubicin and caffeic acid phenethyl ester: design and enhanced antitumor activity
Polymer Journal (2021)