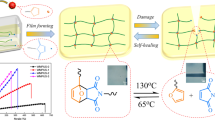
Abstract
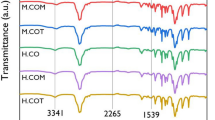
Water-insoluble poly(methacrylic acid) (poly(MAAc)) sponges with nanolayered structures were fabricated via thermal cross-linking with poly(vinyl alcohol) (PVA). The cross-linked water-insoluble sponges contained up to 75 wt% poly(MAAc) when the molecular weight of PVA and the cross-linking time were adjusted appropriately. After immersion in a NaClO·5H2O aqueous solution for 24 h, all poly(MAAc)/PVA_50 wt% sponges with different PVA molecular weights were completely dissolved, and their residual weights were approximately 0%. The molar ratio of NaClO·5H2O was 3.6 times that of the vinyl alcohol units in the sponges. The molecular weight (Mn) and molecular weight distribution (Mw/Mn) of poly(MAAc) observed after immersion in the NaClO·5H2O aqueous solution were similar to those of the original poly(MAAc) (Mn: 46200 g/mol, Mw/Mn: 1.65). In contrast, the gel permeation chromatography (GPC) curves for PVA were shifted to lower molecular weights with increasing NaClO·5H2O concentrations. These results suggested that only the PVA in the poly(MAAc)/PVA sponges was decomposed. Multilayer films of poly(MAAc)/PVA with different physicochemical properties were also fabricated. The first and third layers were made of a poly(MAAc)/PVA_10wt% film, and the second layer was made of a poly(MAAc)/PVA _50wt% sponge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cornwall W. The plastic eaters. Science. 2021;373:36–9.
Korley LTJ, Epps TH III, Helms BA, Ryan AJ. Toward polymer upcycling-adding valueand tackling circularity. Science. 2021;373:66–9.
Stubbins A, Law KL, Muñoz SE, Bianchi TS, Zhu L. Plastics in the earth system. Science. 2021;373:51–5.
Soares CTM, Ek M, Östmark E, Gällstedt M, Karlsson S. Recycling of multi-material multilayer plastic packaging: Current trends and future scenarios. Resour Conserv Recycl. 2022;176:105905.
Qu Z, Xu H, Gu H. Synthesis and biomedical applications of poly((meth)acrylic acid) brushes. ACS Appl Mater Interfaces. 2015;7:14537–51.
Veith C, Diot-Néant F, Miller SA, Allais F. Synthesis and polymerization of bio-based acrylates: a review. Polym Chem. 2020;11:7452–70.
Li Q, Bao Y, Wang H, Du F, Li Q, Jin B, et al. A facile and highly efficient strategy for esterification of poly(meth)acrylic acid with halogenated compounds at room temperature promoted by 1,1,3,3-tetramethylguanidine. Polym Chem. 2013;4:2891–7.
Shevtsov VY, Hsin T-Y, Shieh Y-T. Preparation of amphiphilic copolymers via base-catalyzed hydrolysis of quaternizedpoly[2-(dimethylamino)ethyl methacrylate]. Polym Chem. 2022;13:1429–36.
Elladiou M, Patrickios CS. 2-(Pyridin-2-yl)ethanol asa protecting group for carboxylic acids: chemical and thermal cleavage, andconversion of poly[2-(pyridin-2-yl)ethyl methacrylate] to poly(methacrylicacid). Polym Chem. 2012;3:3228–31.
Hermens JGH, Jensma A, Feringa BL. Highly efficient biobased synthesis of acrylic acid. Angew Chem Int Ed. 2022;61:e202112618.
Fujimoto K, Yamawaki-Ogata A, Narita Y, Kotsuchibashi Y. Fabrication of cationic poly(vinyl alcohol) films cross-linked using copolymers containing quaternary ammonium cations, benzoxaborole, and carboxy groups. ACS Omega. 2021;6:17531–44.
Fujimoto K, Saito A, Kotsuchibashi Y. Cicada-wing-inspired nanopillar hydrogels consisting of poly(vinyl alcohol) and poly(methacrylic acid) for capturing bacteria through their flexibility and wide range of motion. ACS Macro Lett. 2022;11:727–32.
Hassan CM, Peppas NA. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv Polym Sci. 2000;153:37–65.
Chiellini E, Corti A, D’Antone S, Solaro R. Biodegradation of poly(vinyl alcohol) based materials. Prog Polym Sci. 2003;28:963–1014.
DeMerlis CC, Schoneker DR. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem Toxicol. 2003;41:319–26.
Park J-A, Kang J-K, Lee S-C, Kim S-B. Electrospun poly(acrylic acid)/poly(vinyl alcohol) nanofibrous adsorbents for Cu(II) removal from industrial plating wastewater. RSC Adv. 2017;7:18075–84.
Truong YB, Choi J, Mardel J, Gao Y, Maisch S, Musameh M, et al. Macromol Mater Eng. 2017;302:No.1700024.
Su Y-K, Coxwell CM, Shen S, Miller SA. Polyvinyl alcohol modification with sustainable ketones. Polym Chem. 2021;12:4961–73.
Hakuto N, Saito K, Kirihara M, Kotsuchibashi Y. Preparation of cross-linked poly(vinyl alcohol) films from copolymers with benzoxaborole and carboxylic acid groups, and their degradability in an oxidizing environment. Polym Chem. 2020;11:2469–74.
Kirihara M, Okada T, Sugiyama Y, Akiyoshi M, Matsunaga T, Kimura Y. Sodium hypochlorite pentahydrate crystals (NaOCl·5H2O): A convenient and environmentally benign oxidant for organic synthesis. Org Process Res Dev. 2017;21:1925–37.
Kirihara M, Osugi R, Saito K, Adachi K, Yamazaki K, Matsushima R, et al. Sodium hypochlorite pentahydrate as a reagent for the cleavage of trans-cyclic glycols. J Org Chem. 2019;84:8330–6.
Hamad D, Mehrvar M, Dhib R. Experimental study of polyvinyl alcohol degradation in aqueous solution by UV/H2O2 process. Polym Degrad Stab. 2014;103:75–82.
Su Y, Li M, Gao Q, Li X, Ju A, Lu Y, et al. Degradation and kinetic modeling of polyvinyl alcohol in aqueous solutions by a H2O2/Mn(II) system. Fibers Polym. 2017;18:2269–77.
Ali U, Karim KJBA, Buang NA. A review of the properties and applications of poly (methyl methacrylate) (PMMA). Polym Rev. 2015;55:678–705.
Ayre WN, Denyer SP, Evans SL. Ageing and moisture uptake in polymethyl methacrylate (PMMA) bone cements. J Mech Behav Biomed Mater. 2014;32:76–88.
Mengel C, Esker AR, Meyer WH, Wegner G. Preparation and modification of poly(methacrylic acid) and poly(acrylic acid) multilayers. Langmuir 2002;18:6365–72.
Mahmud HNME, Huq AKO, Yahya R. The removal of heavy metal ions from wastewater/ aqueous solution using polypyrrole-based adsorbents: a review. RSC Adv. 2016;6:14778–91.
Zhao G, Huang X, Tang Z, Huang Q, Niu F, Wang X. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: a review. Polym Chem. 2018;9:3562–82.
Acknowledgements
This work was partially supported by the technical research aid projects 2019 (JFE 21st Century Foundation) and research grant 2021 (Iketani Science and Technology Foundation). We are grateful to Nippon Light Metal Co., Ltd., for providing NaClO·5H2O. We are grateful to Mr. Kazuo Hayakawa, Dr. Yusuke Wakikawa, and Dr. Takeaki Koizumi of the Advanced Instrumental Analysis Center at the Shizuoka Institute of Science and Technology for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobayashi, D., Uchida, H., Ishibane, M. et al. Fabrication of thermally cross-linked poly(methacrylic acid)-based sponges with nanolayered structures and their degradation. Polym J 55, 163–170 (2023). https://doi.org/10.1038/s41428-022-00721-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-022-00721-0