Abstract
Background/Objectives
The current study aimed to identify suitable reference miRNA for placental miRNA expression analysis in a set of well-characterized and fetal-sex balanced small- (SGA) and appropriate- (AGA) for gestational age full-term singleton pregnancies.
Subjects/Methods
In this retrospective study, placental samples (n = 106) from 35 SGA (19 male and 16 female) and 71 AGA (30 male and 41 female) full-term singleton pregnancies were utilized. Placental transcript abundance of three widely used reference miRNAs [miR-16-5p and Small nucleolar RNAs (snoRNAs) RNU44 and RNU48] were assessed by real-time quantitative PCR. Raw cycle threshold (Ct) analysis and RefFinder tool analysis were conducted for evaluating stability of expression of these miRNAs.
Results
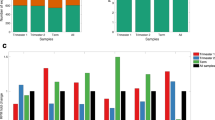
Raw Ct values of miR-16-5p were similar between SGA and AGA births (P = 0.140) and between male and female births within SGA (P = 0.159) and AGA (P = 0.060) births while that of RNU44 and RNU48 were higher in SGA births (P = 0.008 and 0.006 respectively) and in male births within the SGA group (P = 0.005) for RNU44 and in female births within the AGA group (P = 0.048) for RNU48. Across all 106 samples tested using the RefFinder tool, miR-16-5p and RNU44 were equally stable reference miRNAs.
Conclusion
We recommend miR-16-5p and RNU44 as suitable reference miRNAs for placental samples from settings similar to our study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Morales-Prieto DM, Chaiwangyen W, Ospina-Prieto S, Schneider U, Herrmann J, Gruhn B, et al. MicroRNA expression profiles of trophoblastic cells. Placenta 2012;33:725–34.
Kontomanolis EN, Kalagasidou S, Fasoulakis Z. MicroRNAs as potential serum biomarkers for early detection of ectopic pregnancy. Cureus 2018;10:e2344.
Lee ACC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Heal 2013;1:e26–36.
Higashijima A, Miura K, Mishima H, Kinoshita A, Jo O, Abe S, et al. Characterization of placenta-specific microRNAs in fetal growth restriction pregnancy. Prenat Diagn. 2013;33:214–22.
Tang Q, Wu W, Xu X, Huang L, Gao Q, Chen H, et al. miR-141 contributes to fetal growth restriction by regulating PLAG1 expression. PLoS ONE. 2013;8:e58737.
Huang L, Shen Z, Xu Q, Huang X, Chen Q, Li D. Increased levels of microRNA-424 are associated with the pathogenesis of fetal growth restriction. Placenta 2013;34:624–7.
Hromadnikova I, Kotlabova K, Ondrackova M, Pirkova P, Kestlerova A, Novotna V, et al. Expression profile of C19MC microRNAs in placental tissue in pregnancy-related complications. DNA Cell Biol. 2015;34:437–57.
Higuchi R, Fockler C, Dollinger G, Watson R. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Bio/Technol. 1993;11:1026–30.
Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–94.
Bustin SA, Nolan T. Pitfalls of quantitative real- time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15:155–66.
Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–84.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001;25:402–8.
Corral-Vazquez C, Blanco J, Salas-Huetos A, Vidal F, Anton E. Normalization matters: tracking the best strategy for sperm miRNA quantification. Mol Hum Reprod. 2017;23:45–53.
Ling D, Salvaterra PM. Robust RT-qPCR data normalization: validation and selection of internal reference genes during post-experimental data analysis. Lin B, editor. PLoS One. 2011;6:e17762.
Maccani MA, Padbury JF, Marsit CJ. miR-16 and miR-21 expression in the placenta is associated with fetal growth. PLoS ONE. 2011;6:e21210.
Cindrova-Davies T, Herrera EA, Niu Y, Kingdom J, Giussani DA, Burton GJ. Reduced cystathionine γ-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: hydrogen sulfide as a placental vasodilator. Am J Pathol. 2013;182:1448–58.
Thamotharan S, Chu A, Kempf K, Janzen C, Grogan T, Elashoff DA, et al. Differential microRNA expression in human placentas of term intra-uterine growth restriction that regulates target genes mediating angiogenesis and amino acid transport. PLoS ONE. 2017;12:e0176493.
Bratkovič T, Bozič J, Rogelj B. Functional diversity of small nucleolar RNAs. Nucleic Acids Res 2020;48:1627–51.
Mcmahon M, Contreras A, Ruggero D. Small RNAs with big implications: New insights into H/ACA snoRNA function and their role in human disease. Wiley Interdiscip Rev RNA. 2015;6:173–89.
Solayman MHM, Langaee T, Patel A, El-Wakeel L, El-Hamamsy M, Badary O, et al. Identification of suitable endogenous normalizers for qRT-PCR analysis of plasma microRNA expression in essential hypertension. Mol Biotechnol. 2016;58:179–87.
Bryzgunova OE, Zaripov MM, Skvortsova TE, Lekchnov EA, Grigor’eva AE, Zaporozhchenko IA, et al. Comparative study of extracellular vesicles from the urine of healthy individuals and prostate cancer patients. Carter DRF, editor. PLoS ONE.2016;11:e0157566.
Lange T, Stracke S, Rettig R, Lendeckel U, Kuhn J, Schlüter R, et al. Identification of miR-16 as an endogenous reference gene for the normalization of urinary exosomal miRNA expression data from CKD patients. Ray RB, editor. PLoS ONE. 2017;12:e0183435.
Mukhopadhyay A, Thomas T, Bosch RJ, Dwarkanath P, Thomas A, Duggan CP, et al. Fetal sex modifies the effect of maternal macronutrient intake on the incidence of small-for-gestational-age births: a prospective observational cohort study. Am J Clin Nutr. 2018;108:814–20.
WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee.World Health Organization, Geneva, Switzerland. WHO Tech Rep. Ser. 1995;854:1–452.
Mukhopadhyay A, Ravikumar G, Dwarkanath P, Meraaj H, Thomas A, Crasta J, et al. Placental expression of the insulin receptor binding protein GRB10: Relation to human fetoplacental growth and fetal gender. Placenta 2015;36:1225–30.
Wang Y, Lumbers ER, Arthurs AL, Corbisier de Meaultsart C, Mathe A, Avery-Kiejda KA, et al. Regulation of the human placental (pro)renin receptor-prorenin-angiotensin system by microRNAs. Mol Hum Reprod. 2018;24:453–64.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–12.
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper - Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26:509–15.
Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–50.
Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol. 2006;7:33.
De Spiegelaere W, Dern-Wieloch J, Weigel R, Schumacher V, Schorle H, Nettersheim D, et al. Reference gene validation for RT-qPCR, a note on different available software packages. Cotterill S, editor. PLoS ONE. 2015;10:e0122515.
Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, et al. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999;75:291–5.
Mukhopadhyay A, Ravikumar G, Meraaj H, Dwarkanath P, Thomas A, Crasta J, et al. Placental expression of DNA methyltransferase 1 (DNMT1): gender-specific relation with human placental growth. Placenta 2016;48:119–25.
Xie F, Xiao P, Chen D, Xu L, Zhang B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol Biol. 2012;80:75–84.
Wang D, Na Q, Song WW, Song GY. Altered expression of miR-518b and miR-519a in the placenta is associated with low fetal birth weight. Am J Perinatol. 2014;31:729–34.
Tryggestad JB, Vishwanath A, Jiang S, Mallappa A, Teague AM, Takahashi Y, et al. Influence of gestational diabetes mellitus on human umbilical vein endothelial cell miRNA. Clin Sci. 2016;130:1955–67.
Lasabová Z, Vazan M, Zibolenova J, Svecova I. Overexpression of miR-21 and miR-122 in preeclamptic placentas. Neuroendocrinol Lett. 2015;36:695–9.
McDermott AM, Kerin MJ, Miller N. Identification and validation of miRNAs as endogenous controls for RQ-PCR in blood specimens for breast cancer studies. Samant R, editor. PLoS ONE. 2013;8:e83718.
Gee HE, Buffa FM, Camps C, Ramachandran A, Leek R, Taylor M, et al. The small-nucleolar RNAs commonly used for microRNA normalisation correlate with tumour pathology and prognosis. Br J Cancer. 2011;104:1168–77.
Lee DC, Romero R, Kim JS, Tarca AL, Montenegro D, Pineles BL, et al. MiR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am J Pathol. 2011;179:590–602.
Acknowledgements
We thank the pregnant women who participated in the study and doctors and nurses who made this study possible. The contribution of the research assistants Ms. Nancy N, Ms. Roopashree C, Ms. Aruna BS and Ms. Arogya M who collected the samples and data and of histopath technician Ms. Mahalakshmi S assisted the pathologists in placental grossing experiments is acknowledged. This work was supported by the Women Scientist Scheme, Department of Science & Technology, Government of India to PK (Reference no. SR/WOS-A/LS-669/2016) and the Department of Biotechnology, Government of India grants to AM and AVK (Grant sanction nos. BT/PR22326/MED/97/349/2016 and BT/PR30276/MED/97/399/2018).
Author information
Authors and Affiliations
Contributions
AM, AVK and PK designed the study. AM and PK contributed to the planning of the experiments. PD, GR, AT and JC provided samples and clinical information for the study. PK performed all experiments. AM and TT conducted the statistical analyses. AM and PK wrote the manuscript. AM, AVK, TT and PD contributed to the interpretation of the results and manuscript writing. All authors discussed the results, have seen and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kochhar, P., Dwarkanath, P., Ravikumar, G. et al. Placental expression of RNU44, RNU48 and miR-16-5p: stability and relations with fetoplacental growth. Eur J Clin Nutr 76, 722–729 (2022). https://doi.org/10.1038/s41430-021-01003-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-021-01003-3
This article is cited by
-
Identification of stable reference genes in peripheral blood mononuclear cells from type 2 diabetes mellitus patients
Scientific Reports (2023)
-
RefFinder: a web-based tool for comprehensively analyzing and identifying reference genes
Functional & Integrative Genomics (2023)
-
Placental expression of miR-21-5p, miR-210-3p and miR-141-3p: relation to human fetoplacental growth
European Journal of Clinical Nutrition (2022)