Abstract
To comprehensively estimate the association of gestational diabetes mellitus (GDM) risk with maternal red blood cell (RBC) folate, plasma/serum folate, dose and duration of folic acid supplement (FAS) intake and vitamin B12 separately. PubMed, Web of science, CNKI, and Wanfang Databases were searched through March 26, 2021. We synthesized data using random-effects model meta-analysis in Stata 12.0. Sensitivity, subgroup and dose-response analyses were also performed. The certainty of evidence was evaluated using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE). Twenty six datasets from thirteen eligible observational studies were included in the study. We found a significant increase of GDM risk with the highest versus lowest category of RBC folate (OR = 1.96, 95% CI: 1.48–2.61, I2 = 0.0%, moderate-certainty evidence) and plasma/serum folate (OR = 1.23, 1.02–1.48, I2 = 57.8%, low-certainty evidence). The dose-response analysis revealed that each 200 ng/ml increase in RBC folate was significantly associated with 8% higher GDM risk. No significant association between dose of FAS intake and GDM risk was found with very low cetainty. Meanwhile, longer duration (≥3 months) of FAS conferred 56% significant higher GDM risk (OR = 1.56, 1.02–2.39, very low certainty evidence). No significant association of GDM risk with highest plasma/serum B12 was observed compared to lowest B12 (OR = 0.77, 0.58–1.02, very low-certainty evidence). Moderate-certainty evidence suggests that higher RBC folate appears to significantly increase GDM risk. Higher plasma/serum folate may increase GDM risk but with low certainty. Further well-designed trials or prospective studies are needed.
Similar content being viewed by others
Introduction
Gestational diabetes mellitus (GDM) is a condition in which glucose intolerance is of variable severity with onset or first detected during pregnancy resulting in varying degrees of hyperglycemia [1]. The global prevalence of GDM has been in climbing trend and estimated to be 16.9% worldwide in women (20–49 years) varying with population characteristics and the diagnostic criteria used [2, 3]. The pathogenesis of GDM may include genetic susceptibility, exacerbated insulin resistance, chronic inflammatory response, oxidative stress and other aspects [4]. GDM is of relevance to elevated adverse perinatal complications and cardiometabolic risk such as obesity, type 2 diabetes hypertension, hyperlipidemia and lifetime cardiovascular disease in GDM women and their offspring [5,6,7].
A number of factors associated with GDM risk have been implicated, including obesity, excess gestational weight gain, family history of diabetes, physical inactivity, advanced maternal age and genetic factors [8]. It is a critical and urgent issue to identify more modifiable risk factors and promote GDM prevention from a public health and maternal and child health perspective.
Folate and vitamin B12 are water-soluble vitamins and could not be synthesized by the body itself and must be obtained from food. In the daily diet, folate is mainly from green leafy vegetables, fruits, egg yolks, and legumes, while vitamin B12 comes from animal products. Folate and vitamin B12 are key cofactors in one carbon metabolic pathway, which gets involved in DNA methylation and cell metabolism via generating precursors of nucleotide biosynthesis and methyl groups for methylation reactions [9, 10]. With the rising of maternal blood volume and renal blood flow, requirement of folate and vitamin B12 is dramatically elevated during pregnancy and the risk of inadequate intake also arises [11].
Since the early 1990s, folic acid supplement (FAS) has been recommended globally prior to and during the first trimester of pregnancy to prevent neural tube defects (NTDs) [12]. As many women continue to take folic acid supplements beyond the first trimester, the combined effects of supplementation and fortification have resulted in substantial increases in folate concentrations among women of reproductive age [13]. However, the GDM prevalence has been rising continuously. Emerging epidemiological studies reveal that an imbalance of high folate status or intake and low vitamin B12 status during pregnancy predisposes women to elevated GDM risk and their offspring to insulin resistance and adiposity and low birthweight [10, 14]. Chen et al. [15] reported that higher maternal RBC folate and vitamin B12 levels in early pregnancy are significantly associated with GDM risk, while the balance of folate/vitamin B12 is not significantly associated with GDM. Another two studies also showed that higher maternal RBC folate concentrations increase the GDM risk in Chinese population [16, 17]. However, there are inconsistent findings regarding the association of folate status and risk of GDM. Those results are difficult to compare due to the varied dosage and duration of FAS and tested in serum or plasma [18,19,20,21,22,23]. In addition, to our knowledge, only one systematic review included two studies for quantitative analysis and examined the influence of vitamin B12 insufficiency on GDM, however, the studies had small sample sizes and did not exclude the potential confounders [24]. Moreover, the relationship between vitamin B12 and GDM is still unclear and recent studies have conflicting results [15, 25, 26].
Therefore, we conducted a meta-analysis with the following aims: (1) to systematically benchmark current knowledge of the associations of RBC and plasma/serum folate, dose and duration of FAS, vitamin B12 with GDM; (2) to further explore the potential dose-response relationship if sufficient data available; and (3) to assess the robustness of the findings by performing sensitivity analyses and subgroup analyses based on study characteristics.
Methods
We have used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [27].
Search strategy
We comprehensively searched relevant literatures in PubMed, Web of Science, China National Knowledge Infrastructure (CNKI), and Wanfang Databases from inception to March 26, 2021. The following search string was used in PubMed: (“folic acid” [MeSH Terms] OR (folic AND acid) OR folate OR (vitamin AND (B-9 OR B9))) OR ((vitamin AND (B-12 OR B12)) OR cobalamin OR cobalamin [MeSH Terms])) AND (“diabetes, gestational” [MeSH Terms] OR (gestational AND diabetes)). The search string for Web of Science: ((ALL = folate OR ALL = ”folic acid” OR TS = ”folic acid” OR (ALL = folic AND ALL = acid)) OR (ALL = vitamin AND (ALL = B-9 OR ALL = B9)) OR (ALL = vitamin AND (ALL = B-12 OR ALL = B12)) OR ALL = cobalamin) AND (ALL = gestational AND ALL = diabetes). Chinese keywords were used in China National Knowledge Infrastructure (CNKI), and Wanfang Databases, if the articles were in Chinese. Additional articles were also manually searched in bibliographies of retrieved articles and reviews.
Study selection
Inclusion criteria were as following: (1) Reported intake of folate status or vitamin B12 as exposure and in at least two categories; (2) Provided sufficient data for calculation of their effect on GDM. (3) Cohort, case-control and cross-sectional studies were eligible.
Studies were excluded if: (1) Exposure of folate status or vitamin B12 was not evaluated or not directly linked to GDM; (2) Studies were not conducted in population. (3) Reviews, guideline, comment, case reports; (4) Studies were not written in English or Chinese; (5) Duplicate studies or those based on the overlapping data sets, of which those with the largest sample size and most detailed results were included preferentially.
NoteExpress version 3.0.4.6732 was used to merge retrieved citations and eliminate duplications.
Data extraction
Two independent investigators (NN Li, JC Jiang) extracted the following information from eligible studies: tittle, first author, year of publication, study design, country, number of participants/GDM, mean age and or range, exposure of folate status or B12, criteria of GDM assessment and date, effect size, and covariates adjusted. If a study reported several estimates with adjustment for different confounders, results were included in the analyses for the one adjusting for the largest number of covariates. If any data were not available, we attempted to contact the authors for help. Any disagreement were resolved by reaching a consensus with a third independent reviewer (LL Guo).
Quality assessment
The methodological quality of the enrolled cohort/case-control studies were assessed using Newcastle–Ottawa Scale (NOS) [28], and the cross sectional studies using Agency for Healthcare Research and Quality (USA) (AHRQ) [29]. The maximum score of NOS and AHRQ were 9 and 11, respectively. For NOS, score of ≤6, 7–8, and 9 represent low, medium, and high quality, respectively. For AHRQ, score of ≤3, 4–7, and 8–11 represent low, medium, and high quality, respectively.
We also use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to rate the certainty of evidence [30]. The evidence were assessed in five domains for downgrade (risk of bias, imprecision, inconsistency, indirectness, publication bias) and three domains for upgrade (large magnitude of effect, dose-response gradient, all residual confounding would decrease magnitude of effect). We rated the certainty of the evidence as “very low,” “low,” “moderate,” or “high” and prepared evidence profiles.
Statistical analysis
We used odds ratio (OR) with 95% confidence intervals (CI) to pool the association of each exposure with GDM. For the highest versus the lowest category meta-analysis, we adopted the DerSimonian and Laird (D&L) random effects model (if P for heterogeneity <0.05) and Mantel-Haenszel fix effects model (if P for heterogeneity >0.05) to calculate the adjusted ORs and 95% CI [31] using metan command in Stata. Meanwhile, publication bias was detected by funnel plot and evaluated using Egger’s asymmetry test. Heterogeneity among different studies was tested by Cochrane chi-squared test and calculated by I-squared statistics (variation in effect size attributable to heterogeneity). I2 of 25% or less was considered to be low, 26–50% to be moderate, 51–75% to be high, and 76% or more to be very high heterogeneity. To test the potential influence of each study on pooled effect size, “leave-one-out” sensitivity analyses were performed to evaluate the reliability of the results by excluding a single study at a time using metaninf command in STATA. Subgroup analyses were also conducted by number of participants, study design, geographical region, adjusting for history of DM and adjusting for intakes of other nutrients or not. As advised by the guidelines, if the number of pooled studies for specified exposure was not limited (less than ten) or of high heterogeneity, we would perform meta-regression analysis [32, 33].
We conducted dose-response analyses for the association of GDM with increased level of each exposure using a generalized least squares trend estimation [34], according to the methods recommended by Greenland and Longnecker. We extracted the mean level of exposure, the number of participants and cases, and the ORs with 95% CI in each category. If neither median nor mean values were reported, we used the midpoint of the range. If the lowest category was open-ended, the lowest boundary was set to zero. If the highest category was open-ended, we considered the width of that category to be the same as the width of the adjacent category [35]. We examined the potential nonlinear association by modeling each exposure levels using restricted cubic splines with three knots [36]. Non-linearity test was conducted using Wald-type test by testing the null hypothesis that the coefficient of the second spline was equal to zero.
All procedures of meta-analyses were carried out by using the relevant publicly available command in Stata version 12.0 (StataCorp, College Station, TX, USA) with a two-sided P < 0.05 as statistically significant.
Results
Study selection and characteristics
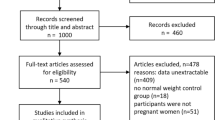
A detailed identification of eligible studies is outlined in Fig. 1. Of the 981 publications retrieved from PubMed, Web of Science, CNKI, and Wanfang Databases, We identified 48 articles by removing the replications and screening on the tittle and abstracts. After reviewing full-text articles, we eventually included 13 eligible studies in present meta-analyses, consisting of ten prospective cohort studies, one case control study and two cross sectional studies.
The characteristics of the 13 studies are presented in Table 1. These 13 individual publications contained 26 eligible datasets including 3 for RBC folate, 8 for plasma folate, 4 for FAS dose, 3 for FAS duration and 8 for plasma/serum vitamin B12. The number of participants involved in 13 studies ranged from 326 to 14553, with mean number of 2420. The incidence of GDM ranged from 3.25% to 18.31% in ten cohorts. The prevalence of GDM in two cross sectional studies were 17.96% and 22.17%. International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria was used as the main method of GDM assessment by 7 of 13 eligible studies. The adjusted ORs were calculated by comparing the highest vs. the lowest level of each exposure adjusting for covariates such as maternal age or BMI plus other factors.
Based on the NOS and AHRQ, All studies were rated of medium to high quality, of which five studies were scored 9, six studies were scored 8 and two studies were scored 7 (Table 1).
RBC folate and GDM risk
As presented in the forest plot (Fig. 2), three eligible studies with no heterogeneity (I2 = 0.0%, P = 0.401) were pooled and a OR of 1.96 (95% CI: 1.48–2.61) was obtained for the risk of GDM comparing the highest versus the lowest category of RBC folate. Owing to dose-response gradient, we rated the certainty of evidence as moderate. The details of the evidence profile for GRADE rating were listed in Supplementary Table 1.
Furthermore, the sensitivity analyses also consistently revealed a significant positive association persisted with the exclusion of each study in turn and confirmed the stability of the results (pooled estimates ranged between 1.91 and 2.34) (Supplementary Fig. 1a). As suggested in subgroup analyses (Table 2), all with no heterogeneity, increased risk of GDM in highest category of RBC folate was seen in subgroups of >1000 participants, <1000 participants, adjusting for history of DM or not, adjusting for intakes of other nutrients or not (pooled effect estimates ranged between 1.72 and 2.47). In addition, no significant differences were found in the risk of GDM in studies that assessed >1000 participants vs. <1000 participants, adjusting for history of DM vs. not, adjusting for intakes of other nutrients vs. not (all Pbetween > 0.05).
Plasma/serum folate and GDM risk
For the highest vs. the lowest category of plasma/serum folate, eight datasets from six studies with high heterogeneity (I2 = 57.8%, P = 0.020) were included in the random-effects meta-analysis (Fig. 2). A significantly positive association of plasma/serum folate with GDM risk were also observed (OR = 1.23 (95% CI: 1.02–1.48). The certainty of evidence was low (Supplementary Table 1).
Similarly, we evaluated the fluctuation of the overall risk of GDM regarding folate status by excluding one study at a time. When excluding the three datasets in turn [15, 23, 25], the association of GDM risk and plasma/serum folate turned to be insignificant, with wide confidence bounds (Supplementary Fig. 1b).
In the subgroup analyses (Table 2), we observed significantly increased risk of GDM with higher plasma/serum folate in the groups of studies with number of participants >1000, or with estimates adjusting for diabetes mellitus history, or with estimates adjusting for intakes of other nutrients, or the Asian population, or the cross-sectional studies. Test for heterogeneity between subgroups may be invalid due to moderate heterogeneity observed in one or more subgroups.
Dose and duration of FAS intake and GDM risk
Due to serious imprecision and inconsistency, very low certainty of evidence showed that women with higher intake of FAS demonstrated a greater risk of GDM but not statistically significant (OR = 1.50, 95% CI: 0.72–3.11), with very high heterogeneity (I2 = 87.4%, P < 0.001). Regarding duration of FAS, as suggested by the meta-analysis of three datasets from two studies with moderate heterogeneity (I2 = 43.7%, P = 0.170), a longer duration (≥3 months) of FAS intake was associated with higher risk of GDM (OR = 1.56, 95% CI: 1.02–2.39) (Fig. 2). Because of poor consistency, the certainty of evidence was categorized as very low (Supplementary Table 1).
Of note, excluding the study conducted in the America by Li et al. [19] removed the observed statistical heterogeneity (I2 = 0.0%, P = 0.928), and we found the highest category of FAS intake had a significantly 2.13 times increased risk of GDM compared to lowest category of FAS (95% CI: 1.52–2.98) (Supplementary Fig. 1c).
Furthermore, the subgroup analyses indicated that the result reported by Li et al. (OR = 0.70, 95% CI: 0.52–0.94) was from western country, and evaluated the relationship between FAS intake during prepregnancy and self-reported GDM, while the other three studies with no heterogeneity (pooled OR = 2.13, 95% CI: 1.52–2.98) were based on Chinese population, the data of FAS intake were collected during pregnancy and the diagnoses of GDM was all in line with IADPSG criteria, which may explain the inconsistent estimates (Table 2) among these studies. By contrast, test for heterogeneity between subgroups of adjusting for history of DM or intakes of other nutrients may be invalid due to considerable heterogeneity observed in one or more subgroups.
Plasma/serum vitamin B12 and GDM risk
With respect to plasma/serum vitamin B12 level, the random-effects meta-analysis pooled eight datasets from six studies with very high heterogeneity (I2 = 77.0%, P < 0.001). No significant association of GDM risk with the highest B12 was observed compared to the lowest B12 (OR = 0.77, 95% CI: 0.58–1.02) (Fig. 2) with very low certainty (Supplementary Table 1), the downgrade was mainly due to serious imprecision and inconsistency.
However, the sensitivity analysis by excluding the study by Chen [15] attenuated the heterogeneity (I2 = 66.0%, P = 0.007) and strengthened this effect, rendering it statistically significant (OR = 0.70, 95% CI: 0.54–0.90). When excluding the other seven datasets in turn, the association of GDM risk and vitamin B12 appeared to be inconclusive (Supplementary Fig. 1e).
In the subgroup analyses (Table 2), we observed significant decreased risk of GDM with higher plasma/serum vitamin B12 in the groups of studies with lower number of participants (<1000), or with estimates adjusting for intakes of other nutrients, or the case control or cross sectional studies.
Dose-response analyses
First, we performed nonlinear dose-response association analyses of GDM risk with RBC folate and FAS using a restricted cubic spine model. The studies and data points on the dose response curve were presented in the Supplementary Table 2. We observed a positive nonlinear association of GDM risk with RBC folate (P nonlinearity = 0.0004; Fig. 3) and FAS intake (P nonlinearity = 0.0002; Fig. 4). Within 500 ng/ml, higher RBC folate was associated with higher GDM risk. When RBC folate >500 ng/ml, then GDM risk appeared not to increase (Fig. 3). The GDM risk appeared to be the lowest in the point of 400 μg/day FAS intake, and when the intake of FAS was up to around 800 μg/day or above, a significant increased risk of GDM began to be observed (Fig. 4).
Then we conducted linear dose-response analyses. The two-stage fixed-effects dose-response model provided the evidence of a linear association of GDM risk with RBC folate, and we found that each 200 ng/ml increase in RBC folate was related to 8% higher risk of GDM (OR = 1.08, 95% CI: 1.03–1.13, n = 3 studies). However, we did not find the linear dose-response association of GDM risk with FAS intake (OR = 1.04, 95% CI: 0.94–1.15, P = 0.457, n = 3 studies, for each 100 μg/day increment in FAS intake).
In addition, we did not conduct dose-response analyses due to a lack of sufficient data regarding each category of plasma/serum folate, duration of FAS and plasma/serum vitamin B12.
Publication bias
For each meta-analysis including less than ten studies, the minimum number of studies suggested for use of funnel plot [32, 37], the power of publication bias assessment may be too low to distinguish true bias from chance. As presented in Supplementary Fig. 2, there was no evidence of publication bias for RBC folate (Egger’s test P = 0.745, Begg’s test P = 0.602), FAS intake (Egger’s test P = 0.367, Begg’s test P = 0.497), FAS duration (Egger’s test P = 0.585, Begg’s test P = 0.602). The evidence of publication bias was inconclusive for plasma/serum folate (Egger’s test P = 0.009, Begg’s test P = 0.458) and plasma/serum vitamin B12 (Egger’s test P = 0.139, Begg’s test P = 0.013).
Discussions
We systematically conducted the meta-analyses to evaluate the association of GDM risk with one-carbon metabolism-related B vitamins (RBC and plasma/serum folate, dose and duration of FAS intake, vitamin B12) based on thirteen observational studies with 31459 participants and 2766 GDM cases. We found a 96% and 23% significant increase risk of GDM with the highest versus the lowest category of RBC folate and plasma/serum folate, with moderate and low certainty, respectively. The dose-response analysis also revealed that each 200 ng/ml increase in RBC folate was associated with 8% significant higher risk of GDM. No significant increased risk of GDM was observed in the highest vs. the lowest category of FAS intake. A longer duration (≥3 months) of FAS intake was significantly associated with 56% higher risk of GDM. However, due to the very low certainty evidence—downgraded for serious inconsistency, it is uncertain of this effect. In addition, no statistically significant association of GDM risk with the highest plasma/serum B12 level was observed in comparison with the lowest B12.
Because estimating natural dietary intake of folate is difficult and imprecise, RBC and plasma/serum folate, as the biomarkers, are more useful and reliable for evaluating the folate nutritional status. Generally in accordance with the results of a previous meta-analysis [16] without distinguishing specified folate status, we observed similar patterns of increasing GDM risk in relation to excess RBC and plasma/serum folate, respectively. Intriguingly, Chen also reported that the decrease in serum folate from early to midgestation was negatively associated with GDM risk (aOR = 0.95, 95% CI:0.90–0.99), which further reinforced the detrimental effect of excess folate on GDM [15].
As the biomarker of folate status, plasma/serum folate is an indicator of recent folate intake and is dramatically affected by FAS [38]. By comparison RBC folate responds slower to changes in folate intake and represents the long-term folate status [38]. But RBC and plasma/serum folate concentrations are highly correlated. Furthermore, their correlation was reported to be modified by BMI and genotype and substantially by low plasma vitamin B-12 [39]. Previous studies in various populations have consistently shown that NTD risk decreased with increasing RBC folate concentration in relation to dietary folate intake levels. How and to what extent FAS has impact on maternal RBC and plasma/serum folate concentrations still remains uncertain. An ancillary study within the Folic Acid Clinical Trial (FACT) has found that high-dose FAS in early pregnancy increased maternal serum folate but not RBC folate concentrations, suggesting tissue saturation [40]. Notably, a randomized controlled trial in Malaysia indicated that weekly iron-folic acid (IFA) supplements containing 2.8 mg folic acid increased RBC folate and had a lower risk of NTD more than those containing 0.4 mg folate [41]. So it is an interesting and important problem to identify the mutual relation among daily folic acid intake, RBC and plasma/serum folate concentrations, further assess and establish optimal RBC and plasma/serum folate for NTD prevention and recommend the daily total folic acid intake in various population [42]. However, the exact cut-offs to classify clinical and subclinical deficiency or excess remain debated. Therefore, based on the results of our meta-analyses, we anticipate that optimal RBC and plasma/serum folate should be evaluated and figured out in consideration of the potential risk of GDM as well as other adverse effects.
In contrast with the positive associations of GDM risk with RBC and plasma/serum folate, the adverse effect of higher FAS intake on GDM was observed but not statistically significant. However, when we performed the sensitivity and subgroup analysis by excluding the study conducted by Li et al. [19], heterogeneity of the other three Chinese cohort studies disappeared and a significant 2.13 times increased risk of GDM was obtained with the highest vs. the lowest category of FAS intake. The study excluded was based on western population and focused on the prepregnancy intake of FAS and self-reported GDM, which may account for the heterogeneity. In addition, inconsistent with the results of Li et al., Zhu reminded that an increased risk of GDM was not apparent for women using FAS before pregnancy alone [18]. To add, we did not question the adequate intake of FAS, with the reason that we only observed a significant increased risk of GDM when the intake of FAS was up to around 800 μg/day or above as revealed by the non-linear dose-response analysis.
Concomitantly, we found that the significant positive relationship between duration of FAS and GDM risk. However, only two eligible studies both based on Chinese cohorts were included in the analysis and one of them [21] investigated FAS duration before and after pregnancy separately and the other [22] focused on FAS duration before pregnancy alone or in the second trimester alone or the total duration from prepregnancy to early pregnancy separately, which made the results mixed. Notably, In combination of dose and duration of FAS, Qian Li et al. [20] found that FAS above 800 μg/day for long duration (continuous at least 4 weeks prepregnancy and continued for at least 16 weeks during pregnancy before OGTT) was related to the highest GDM risk compared with nonusers in a Chinese population, indicating an additive effect exists. In addition to GDM risk, increasing evidence was reported that high-dose FAS intake during pregnancy may contribute to the rise in gestational hypertension [43, 44] and in adverse outcomes of the offspring, such as allergic disease and autism spectrum disorder [45, 46]. Thus, this highlights the importance of systematically confirming the potential adverse effects of excess dose and duration of FAS intake with differentiating between prepregnancy and other trimester periods separately in various population with larger sample and optimizing the FAS strategy in further studies.
Similar significant shift of risk estimates also occurred in regard to plasma/serum vitamin B12. After excluding the study by Chen et al. [15] in the sensitivity analysis, we observed a significant 30% decrease in GDM risk in the highest categories of plasma/serum B12 compared with the lowest B12, along with reduced heterogeneity. It is reported that elevated maternal vitamin B12 requirements and low serum vitamin B12 concentrations associate with physiological changes during pregnancy [47]. High serum vitamin B12 levels in some disorders such as liver disease may mask functional B12 deficiency [48]. We assumed the discrepancy of the GDM-B12 relationship may be partially explained by the different physiological status and the time points of plasma/serum vitamin B12 measurement between the studies. Furthermore, when excluding the study without adjusting for intakes of other nutrient, the protective effect of plasma/serum B12 on GDM risk turned to be significant. We speculated that the effect of plasma/serum B12 on GDM may be confounded by other factors such as energy and overall nutrients intakes, iron, folate, multivitamin and other supplements. Of note, The highest ORs of GDM were observed among women with combined vitamin B12 insufficiency and high folate concentration [23, 25]. Furthermore, the synergistic adverse effect of imbalance of plasma/serum folate and vitamin B12 (higher folate/B12) on GDM risk was also reported to be further elevated by older maternal age and higher prepregnancy BMI with significant additive interaction, and the effect of folate on GDM could be confounded by serum vitamin B12 levels [25]. The modified effect of age may be explained by the age-related decline in vitamin B12 absorption despite adequate intake in older adults [10]. Although there are not enough data on the interactions between folate and vitamin B12 imbalance and effect modifiers on the risk of GDM, the findings give a promising direction to understand its mechanism and have potential implications for antenatal supplement recommendations. Further studies are needed to confirm the relationship between GDM risk and the imbalance of folate and vitamin B12 from prepregnancy through pregnancy and effect modifiers such as age, BMI and the presence of other nutrients.
Mechanism of the effect
The underlying mechanism of the possible adverse effect of excess folate on GDM remains to be elucidated. The imbalance between folate and vitamin B12 may be one of the possible explanations. Folate and vitamin B12 are key cofactors in one carbon metabolic pathway. One carbon pathway generates precursors for nucleotide biosynthesis and methyl groups for methylation reactions [10]. The deficiency of folate and vitamin B12 may lead to disruption of DNA synthesis, cellular inflammation, adiposity dysfunction with increased lipogenesis and homocysteine levels, which might lead to glucose intolerance and anemia [49]. Furthermore, sufficient folate status could mask the vitamin B12 deficiency, exacerbate the deleterious effect and clinical manifestations of vitamin B12 deficiency with increasing dose of folate and might participate in the pathogenesis of GDM through worsening insulin resistance [50]. Recent study also revealed that low vitamin B12 levels in pregnancy plays a role in epigenetic regulation by altering adipose-derived circulating miRNAs during adipocyte differentiation and resulted in an adverse insulin resistance phenotype [51]. In addition, high folic acid intake and excess unmetabolized plasma folate may get involved in the developing of GDM by reducing natural killer cell cytotoxicity and immune function [52, 53].
Strengths and limitations
The systematical meta-analyses have several strengths. The majority of the included studies were high-quality cohort studies and the pooled analyses were based on risk estimates adjusted for confounding covariates, which strengthen the conclusion. Moreover, we examined strictly methodological quality of the evidence using NOS and AHRQ and assessed the certainty of the evidence via GRADE tool. In addition, we conducted sensitivity analysis, subgroup analysis and dose-response analysis to shed light on the true associations with consideration of potential heterogeneity. In terms of RBC folate, the consistent results of the multiple subgroup analyses and dose-response gradient reinforce its effect on GDM risk and upgrade the certainty of evidence.
Inevitably, we acknowledge several limitations. First, the relatively limited number of eligible studies for each exposure (<10 studies) may make it difficult and less power to safely detect potential bias or sources of heterogeneity. We attempted to address this issue by using random-effect model and subgroup analyses. Secondly, comprehensive dose-response analyses were not conducted as planned due to a lack of sufficient data regarding each category of plasma/serum folate, duration of FAS and plasma/serum vitamin B12. Thirdly, as to the effect of dose and duration of FAS and plasma/serum vitamin B12 on GDM risk, considering serious imprecision, inconsistency, and unexplained heterogeneity, the certainty of evidence remain very low. The results should be interpreted with caution.
So further high-quality longitudinal studies or randomized controlled trials are warranted to clarify the true independent relationships. Furthermore, future studies should measure FAS intake, vitamin B12 and their biomarkers at different pregnancy stages, and adjust the confounding factors such as intakes of other nutrient, age, BMI, history of diabetes mellitus and other covariates in various population.
Conclusions
To conclude, moderate-certainty evidence of observational studies indicates that higher RBC folate appears to be significantly associated with an increasing risk of GDM. The effect across multiple prespecified subgroups and a dose response effect reinforce this conclusion. Higher plasma/serum folate may be significantly associated with increased GDM risk at low certainty. For other comparisons, the certainty of evidence was rated as “very low”, which means that the associations of GDM risk with dose or duration of FAS, maternal plasma/serum vitamin B12 remain unclear. More well-designed studies are needed to confirm the associations.
References
Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018;29:743–54.
Ferrara A. Increasing Prevalence of Gestational Diabetes Mellitus: a public health perspective. Diabetes Care. 2007;30:S141–6.
Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pr. 2014;103:176–85.
Catalano PM. Trying to understand gestational diabetes. Diabet Med. 2014;31:273–81.
Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–9.
Li Z, Cheng Y, Wang D, Chen H, Chen H, Ming W, et al. Incidence rate of type 2 diabetes mellitus after gestational diabetes mellitus: a systematic review and meta-analysis of 170,139 women. J Diabetes Res. 2020;2020:1–12.
Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62:905–14.
Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG.2017;124:804–13.
Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3:21–38.
Paul L, Selhub J. Interaction between excess folate and low vitamin B12 status. Mol Asp Med. 2017;53:43–7.
Wang S, Wang H, Song Y, Ji Y. The role of vitamin b12 in the pathogenesis of gestational diabetes. Acta Microsc. 2020;29:838–45.
Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FAR, et al. Folic acid supplementation for the prevention of neural tube defects: US preventive services task force recommendation statement. JAMA: J Am Med Assoc. 2017;317:183–9.
Best K, Green TJ. Adequate maternal pre-conceptional folate status may reduce the risk of gestational diabetes mellitus. Evid Based Nurs. 2020;2019:103157.
Gadgil M, Joshi K, Pandit A, Otiv S, Joshi R, Brenna JT, et al. Imbalance of folic acid and vitamin B12 is associated with birth outcome: an Indian pregnant women study. Eur J Clin Nutr. 2014;68:726–9.
Chen X, Zhang Y, Chen H, Jiang Y, Wang Y, Wang D, et al. Association of maternal folate and vitamin b12 in early pregnancy with gestational diabetes mellitus: a prospective cohort study. Diabetes Care. 2021;44:217–23.
Xie K, Xu P, Fu Z, Gu X, Li H, Cui X, et al. Association of maternal folate status in the second trimester of pregnancy with the risk of gestational diabetes mellitus. Food Sci Nutr. 2019;7:3759–65.
Liu PJ, Liu Y, Ma L, Yao AM, Chen XY, Hou YX, et al. Associations between gestational diabetes mellitus risk and folate status in early pregnancy and MTHFR C677T polymorphisms in chinese women. Diabetes Metab Syndr Obes. 2020;13:1499–507.
Zhu B, Ge X, Huang K, Mao L, Yan S, Xu Y, et al. Folic acid supplement intake in early pregnancy increases risk of gestational diabetes mellitus: evidence from a prospective cohort study. Diabetes Care. 2016;39:e36–7.
Li M, Li S, Chavarro JE, Gaskins AJ, Ley SH, Hinkle SN, et al. Prepregnancy habitual intakes of total, supplemental, and food folate and risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Care. 2019;42:1034–41.
Li Q, Zhang Y, Huang L, Zhong C, Chen R, Zhou X, et al. High-Dose folic acid supplement use from prepregnancy through midpregnancy is associated with increased risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Care. 2019;42:e113–5.
Cheng G, Sha T, Gao X, He Q, Wu X, Tian Q, et al. The associations between the duration of folic acid supplementation, gestational diabetes mellitus, and adverse birth outcomes based on a birth cohort. Int J Env Res Pub He. 2019;16:4511.
Huang L, Yu X, Li L, Chen Y, Yang Y, Yang Y. Duration of periconceptional folic acid supplementation and risk of gestational diabetes mellitus. Asia Pac J Clin Nutr. 2019;28:321–329.
Lai JS, Pang WW, Cai S, Lee YS, Chan J, Shek L, et al. High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin Nutr. 2018;37:940–7.
Kouroglou E, Anagnostis P, Daponte A, Bargiota A. Vitamin B12 insufficiency is associated with increased risk of gestational diabetes mellitus: a systematic review and meta-analysis. Endocrine. 2019;66:149–56.
Li S, Hou Y, Yan X, Wang Y, Shi C, Wu X, et al. Joint effects of folate and vitamin B12 imbalance with maternal characteristics on gestational diabetes mellitus. J Diabetes. 2019;11:744–51.
Jankovic-Karasoulos T, Furness DL, Leemaqz SY, Dekker GA, Grzeskowiak LE, Grieger JA, et al. Maternal folate, one-carbon metabolism and pregnancy outcomes. Matern Child Nutr. 2021;17:e13064.
Arya S, Kaji AH, Boermeester MA. PRISMA reporting guidelines for meta-analyses and systematic reviews. Jama Surg. 2021;156:789–90.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–05.
Chou R, Baker WL, Bañez LL, Iyer S, Myers ER, Newberry S, et al. Agency for Healthcare Research and Quality Evidence-based Practice Center methods provide guidance on prioritization and selection of harms in systematic reviews. J Clin Epidemiol. 2018;98:98–104.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ.2008;336:924–6.
Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Cornell JE, Liao JM, Stack CB, Mulrow CD. Annals understanding clinical research: evaluating the meaning of a summary estimate in a meta-analysis. Ann Intern Med. 2017;167:275–7.
Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020;35:49–60.
Sander G, Longnecker MP. Methods for trend estimation from summarized Dose-Response data, with applications to Meta-Analysis. Am J Epidemiol. 1992;135:1301–9.
Qiang Y, Li Q, Xin Y, Fang X, Tian Y, Ma J, et al. Intake of dietary One-Carbon Metabolism-Related b vitamins and the risk of esophageal cancer: a dose-response meta-analysis. Nutrients.2018;10:835.
Harrell FJ, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–202.
Fellow JPHS, Altman DG. Assessing risk of bias in included studies[M]. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series, 2011.
Nichol CharlesA. Folates and pterins, vol. 3; Nutritional, pharmacological and physiological aspects. Neurochem Int. 1987;11:479.
Chen M, Rose CE, Qi YP, Williams JL, Yeung LF, Berry RJ, et al. Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am J Clin Nutr. 2019;109:1452–61.
Murphy M, Muldoon KA, Sheyholislami H, Behan N, Lamers Y, Rybak N, et al. Impact of high-dose folic acid supplementation in pregnancy on biomarkers of folate status and 1-carbon metabolism: an ancillary study of the Folic Acid Clinical Trial (FACT). Am J Clin Nutr. 2021;113:1361–71.
Samson KLI, Loh SP, Lee SS, Sulistyoningrum DC, Khor GL, Shariff ZBM, et al. Weekly iron–folic acid supplements containing 2.8 mg folic acid are associated with a lower risk of neural tube defects than the current practice of 0.4 mg: a randomised controlled trial in Malaysia. BMJ Glob Health. 2020;5:e3897.
Crider KS, Qi Y, Devine O, Tinker SC, Berry RJ. Modeling the impact of folic acid fortification and supplementation on red blood cell folate concentrations and predicted neural tube defect risk in the United States: Have we reached optimal prevention? Am J Clin Nutr. 2018;107:1027–34.
Li Q, Xu S, Chen X, Zhang X, Li X, Lin L, et al. Folic acid supplement use and increased risk of gestational hypertension. Hypertension.2020;76:150–6.
Oken E, Ning Y, Rifas-Shiman SL, Rich-Edwards JW, Olsen SF, Gillman MW. Diet during pregnancy and risk of preeclampsia or gestational hypertension. Ann Epidemiol. 2007;17:663–8.
Mcstay C, Prescott S, Bower C, Palmer D. Maternal folic acid supplementation during pregnancy and childhood allergic disease outcomes: a question of timing? Nutrients. 2017;9:123.
Raghavan R, Riley AW, Volk H, Caruso D, Hironaka L, Sices L, et al. Maternal multivitamin intake, plasma folate and vitamin b12 levels and autism spectrum disorder risk in offspring. Paediatr Perinat Ep. 2018;32:100–11.
Looman M, Geelen A, Samlal R, Heijligenberg R, Klein GJ, Balvers M, et al. Changes in micronutrient intake and status, diet quality and glucose tolerance from preconception to the second trimester of pregnancy. Nutrients. 2019;11:460.
Solomon LR. Disorders of cobalamin (Vitamin B12) metabolism: emerging concepts in pathophysiology, diagnosis and treatment. Blood Rev. 2007;21:113–30.
Palmer AM, Kamynina E, Field MS, Stover PJ. Folate rescues vitamin B12 depletion-induced inhibition of nuclear thymidylate biosynthesis and genome instability. Proc Natl Acad Sci. 2017;114:E4095–102.
Selhub J, Morris MS, Jacques PF. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci USA. 2007;104:19995–20000.
Adaikalakoteswari A, Vatish M, Alam MT, Ott S, Kumar S, Saravanan P. Low vitamin b12 in pregnancy is associated with Adipose-Derived circulating miRs targeting PPARγ and insulin resistance. J Clin Endocrinol Metab. 2017;102:4200–09.
Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136:189–94.
Sawaengsri H, Wang J, Reginaldo C, Steluti J, Wu D, Meydani SN, et al. High folic acid intake reduces natural killer cell cytotoxicity in aged mice. J Nutr Biochem. 2016;30:102–7.
Acknowledgements
Many thanks to Professor Ling Wang and Dr. Songyuan Deng for the valuable suggestions on the revision of the article.
Author information
Authors and Affiliations
Contributions
NL developed the idea and study design; NL and JJ carried out the literature searching, data-extracting and critical appraisal; NL, JJ and LG performed the statistical analyses and interpretation; NL drafted and edited the manuscript; JJ and LG gave advice on extensive revision. All authors have read and approved the submission of final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, N., Jiang, J. & Guo, L. Effects of maternal folate and vitamin B12 on gestational diabetes mellitus: a dose-response meta-analysis of observational studies. Eur J Clin Nutr 76, 1502–1512 (2022). https://doi.org/10.1038/s41430-022-01076-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-022-01076-8