Abstract
Background/Objectives
To analyse retinal nerve fibre layer (RNFL) defect measurements obtained from red-free fundus photography and optical coherence tomography (OCT) en face imaging, respectively, and to compare them for the strength of the structure–function association.
Subjects/Methods
Two hundred and fifty-six glaucomatous eyes of 256 patients with localized RNFL defect on red-free fundus photography were enrolled. A subgroup analysis included 81 highly myopic eyes (≤ –6.0 dioptres). Angular width of RNFL defect was compared between red-free fundus photography (i.e., red-free RNFL defect) and OCT en face imaging (i.e., en face RNFL defect). The correlation between angular width of each RNFL defect and functional outcomes, reported as mean deviation (MD) and pattern standard deviation (PSD), were assessed and compared.
Results
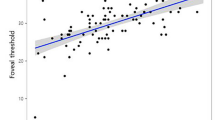
The angular width of en face RNFL defect was measured smaller than that of red-free RNFL defect in 91.0% eyes (mean difference, 19.98°). The association of en face RNFL defect with MD and PSD was stronger (R2 = 0.311 and R2 = 0.372, respectively) than that of red-free RNFL defect with MD and PSD (R2 = 0.162 and R2 = 0.137, respectively) (P < 0.05 for all). Especially in highly myopic eyes, the association of en face RNFL defect with MD and PSD was much stronger (R2 = 0.503 and R2 = 0.555, respectively) than that of red-free RNFL defect with MD and PSD (R2 = 0.216 and R2 = 0.166, respectively) (P < 0.05 for all).
Conclusions
En face RNFL defect showed a higher correlation with severity of visual field loss than did red-free RNFL defect. The same dynamic was observed for highly myopic eyes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Sommer A, Quigley HA, Robin AL, Miller NR, Katz J, Arkell S. Evaluation of nerve fiber layer assessment. Arch Ophthalmol (Chic, Ill: 1960). 1984;102:1766–71.
Jonas JB, Dichtl A. Evaluation of the retinal nerve fiber layer. Surv Ophthalmol. 1996;40:369–78.
Quigley HA, Addicks EM. Quantitative studies of retinal nerve fiber layer defects. Arch Ophthalmol (Chic, Ill: 1960). 1982;100:807–14.
Quigley HA. Examination of the retinal nerve fiber layer in the recognition of early glaucoma damage. Trans Am Ophthalmol Soc. 1986;84:920–66.
Kim JM, Park KH, Kim SJ, Jang HJ, Noh E, Kim MJ, et al. Comparison of localized retinal nerve fiber layer defects in highly myopic, myopic, and non-myopic patients with normal-tension glaucoma: a retrospective cross-sectional study. BMC Ophthalmol. 2013;13:67.
Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–64.
Alluwimi MS, Swanson WH, Malinovsky VE, King BJ. Customizing Perimetric Locations Based on En Face Images of Retinal Nerve Fiber Bundles With Glaucomatous Damage. Transl Vis Sci Technol. 2018;7:5.
Hood DC, Fortune B, Mavrommatis MA, Reynaud J, Ramachandran R, Ritch R, et al. Details of Glaucomatous Damage Are Better Seen on OCT En Face Images Than on OCT Retinal Nerve Fiber Layer Thickness Maps. Investig Ophthalmol Vis Sci. 2015;56:6208–16.
Jung JH, Park JH, Yoo C, Kim YY. Localized Retinal Nerve Fiber Layer Defects in Red-free Photographs Versus En Face Structural Optical Coherence Tomography Images. J Glaucoma. 2018;27:269–74.
Park JH, Yoo C, Kim YY. Localized Retinal Nerve Fiber Layer Defect Location Among Red-free Fundus Photographs, En Face Structural Images, and Cirrus HD-OCT Maps. J Glaucoma. 2019;28:1054–60.
Ji MJ, Park JH, Yoo C, Kim YY. Comparison of the Progression of Localized Retinal Nerve Fiber Layer Defects in Red-free Fundus Photograph, En Face Structural Image, and OCT Angiography Image. J Glaucoma. 2020;29:698–703.
Lim AB, Park JH, Jung JH, Yoo C, Kim YY. Characteristics of diffuse retinal nerve fiber layer defects in red-free photographs as observed in optical coherence tomography en face images. BMC Ophthalmol. 2020;20:16.
Kim YK, Choi HJ, Jeoung JW, Park KH, Kim DM. Five-year incidence of primary open-angle glaucoma and rate of progression in health center-based Korean population: the Gangnam Eye Study. PloS one. 2014;9:e114058.
Prum BE, Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, et al. Primary Open-Angle Glaucoma Preferred Practice Pattern® Guidelines. Ophthalmology. 2016;123:P41–P111.
Hoyt WF, Frisén L, Newman NM. Fundoscopy of nerve fiber layer defects in glaucoma. Investigative Ophthalmol. 1973;12:814–29.
Nukada M, Hangai M, Mori S, Nakano N, Nakanishi H, Ohashi-Ikeda H, et al. Detection of localized retinal nerve fiber layer defects in glaucoma using enhanced spectral-domain optical coherence tomography. Ophthalmology. 2011;118:1038–48.
Kimura Y, Hangai M, Morooka S, Takayama K, Nakano N, Nukada M, et al. Retinal nerve fiber layer defects in highly myopic eyes with early glaucoma. Investigative Ophthalmol Vis Sci. 2012;53:6472–8.
Rosenfeld PJ, Durbin MK, Roisman L, Zheng F, Miller A, Robbins G, et al. ZEISS Angioplex™ Spectral Domain Optical Coherence Tomography Angiography: Technical Aspects. Dev Ophthalmol. 2016;56:18–29.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Malik R, Swanson WH, Garway-Heath DF. ‘Structure-function relationship’ in glaucoma: past thinking and current concepts. Clin Exp Ophthalmol. 2012;40:369–80.
Bizios D, Heijl A, Bengtsson B. Integration and fusion of standard automated perimetry and optical coherence tomography data for improved automated glaucoma diagnostics. BMC Ophthalmol. 2011;11:20.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol (Chic, Ill: 1960). 2002;120:1268–79.
Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I. Results of the European glaucoma prevention study. Ophthalmology. 2005;112:366–75.
Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Investigative Ophthalmol Vis Sci. 2002;43:2213–20.
El Beltagi TA, Bowd C, Boden C, Amini P, Sample PA, Zangwill LM, et al. Retinal nerve fiber layer thickness measured with optical coherence tomography is related to visual function in glaucomatous eyes. Ophthalmology. 2003;110:2185–91.
Ferreras A, Pablo LE, Garway-Heath DF, Fogagnolo P, García-Feijoo J. Mapping standard automated perimetry to the peripapillary retinal nerve fiber layer in glaucoma. Investigative Ophthalmol Vis Sci. 2008;49:3018–25.
Aptel F, Sayous R, Fortoul V, Beccat S, Denis P. Structure-function relationships using spectral-domain optical coherence tomography: comparison with scanning laser polarimetry. Am J Ophthalmol. 2010;150:825–33.
Leite MT, Zangwill LM, Weinreb RN, Rao HL, Alencar LM, Medeiros FA. Structure-function relationships using the Cirrus spectral domain optical coherence tomograph and standard automated perimetry. J Glaucoma. 2012;21:49–54.
Raza AS, Cho J, de Moraes CG, Wang M, Zhang X, Kardon RH, et al. Retinal ganglion cell layer thickness and local visual field sensitivity in glaucoma. Arch Ophthalmol (Chic, Ill: 1960). 2011;129:1529–36.
Rao HL, Zangwill LM, Weinreb RN, Leite MT, Sample PA, Medeiros FA. Structure-function relationship in glaucoma using spectral-domain optical coherence tomography. Arch Ophthalmol (Chic, Ill: 1960). 2011;129:864–71.
Sommer A, D’Anna SA, Kues HA, George T. High-resolution photography of the retinal nerve fiber layer. Am J Ophthalmol. 1983;96:535–9.
Vermeer KA, van der Schoot J, Lemij HG, de Boer JF. RPE-normalized RNFL attenuation coefficient maps derived from volumetric OCT imaging for glaucoma assessment. Investigative Ophthalmol Vis Sci. 2012;53:6102–8.
Miura N, Omodaka K, Kimura K, Matsumoto A, Kikawa T, Takahashi S, et al. Evaluation of retinal nerve fiber layer defect using wide-field en-face swept-source OCT images by applying the inner limiting membrane flattening. PloS one. 2017;12:e0185573.
Sakamoto M, Mori S, Ueda K, Kurimoto T, Kusuhara S, Yamada-Nakanishi Y, et al. En Face Slab Images Visualize Nerve Fibers With Residual Visual Sensitivity in Significantly Thinned Macular Areas of Advanced Glaucomatous Eyes. Investigative Ophthalmol Vis Sci. 2019;60:2811–21.
Christopher M, Bowd C, Belghith A, Goldbaum MH, Weinreb RN, Fazio MA, et al. Deep Learning Approaches Predict Glaucomatous Visual Field Damage from OCT Optic Nerve Head En Face Images and Retinal Nerve Fiber Layer Thickness Maps. Ophthalmology. 2020;127:346–56.
Leung CK, Mohamed S, Leung KS, Cheung CY, Chan SL, Cheng DK, et al. Retinal nerve fiber layer measurements in myopia: An optical coherence tomography study. Investigative Ophthalmol Vis Sci. 2006;47:5171–6.
Mwanza JC, Durbin MK, Budenz DL, Sayyad FE, Chang RT, Neelakantan A, et al. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology. 2012;119:1151–8.
Schuman JS. Optical Coherence Tomography in High Myopia. JAMA Ophthalmol. 2016;134:1040–40.
Leung CK, Yu M, Weinreb RN, Mak HK, Lai G, Ye C, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: interpreting the RNFL maps in healthy myopic eyes. Investigative Ophthalmol Vis Sci. 2012;53:7194–200.
Yamashita T, Kii Y, Tanaka M, Yoshinaga W, Yamashita T, Nakao K, et al. Relationship between supernormal sectors of retinal nerve fibre layer and axial length in normal eyes. Acta Ophthalmol. 2014;92:e481–7.
Leung CK, Chong KK, Chan WM, Yiu CK, Tso MY, Woo J, et al. Comparative study of retinal nerve fiber layer measurement by StratusOCT and GDx VCC, II: structure/function regression analysis in glaucoma. Investigative Ophthalmol Vis Sci. 2005;46:3702–11.
Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710.
Harwerth RS, Vilupuru AS, Rangaswamy NV, Smith EL 3rd. The relationship between nerve fiber layer and perimetry measurements. Investigative Ophthalmol Vis Sci. 2007;48:763–73.
Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Investigative Ophthalmol Vis Sci. 2000;41:1774–82.
Swanson WH, Felius J, Pan F. Perimetric defects and ganglion cell damage: interpreting linear relations using a two-stage neural model. Investigative Ophthalmol Vis Sci. 2004;45:466–72.
Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol (Chic, Ill: 1960). 2002;120:701–13.
Saunders LJ, Russell RA, Crabb DP. The Coefficient of Determination: What Determines a Useful R 2 Statistic? Investigative Ophthalmol Vis Sci. 2012;53:6830–2.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2019R1F1A1058426).
Author information
Authors and Affiliations
Contributions
Conceptualisation, methodology: HJC Data curation, formal analysis, methodology: EB Writing original draft: EB Visualisation: EB Writing-review & editing: EB, HJC.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bak, E., Choi, H.J. Structure-function relationship in glaucoma: Optical coherence tomography en face imaging vs. red-free fundus photography. Eye 37, 2969–2976 (2023). https://doi.org/10.1038/s41433-023-02452-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02452-9