Abstract
Discordance between different genomic regions, often identified through multilocus sequencing of selected markers, presents particular difficulties in identifying historical processes which drive species diversity and boundaries. Mechanisms causing discordance, such as incomplete lineage sorting or introgression due to interspecific hybridization, are better identified based on population-level genomic datasets. In the toads of the Rhinella granulosa species group, patterns of mito-nuclear discordance and potential hybridization have been reported by several studies. However, these patterns were proposed based on few loci, such that alternative mechanisms behind gene-tree heterogeneity cannot be ruled out. Using genome-wide ddRADseq loci from a subset of species within this clade, we found only partial concordance between currently recognized species-level taxon boundaries and patterns of genetic structure. While most taxa within the R. granulosa group correspond to clades, genetic clustering analyses sometimes grouped distinct taxonomic units into a single cluster. Moreover, levels of admixture between inferred clusters were limited and restricted to a single taxon pair which is best explained by incomplete lineage sorting as opposed to introgressive hybridization, according to D-statistics results. These findings contradict previous assertions of widespread cryptic diversity and gene flow within the R. granulosa clade. Lastly, our analyses suggest that diversification events within the Rhinella granulosa group mostly dated back to the early Pliocene, being generally younger than species divergences in other closely related clades that present high levels of cross-species gene flow. This finding uniquely contradicts common assertions that this young clade of toads exhibits interspecific hybridization.
Similar content being viewed by others
Introduction
Discordance between different genomic regions has been increasingly observed in numerous species over the past decades (Firneno et al. 2020; Ivanov et al. 2018; Toews and Brelsford 2012). This phenomenon has been explored more recently with high throughput sequencing, allowing the ability to analyze the nuclear genome at a larger scale compared to previous single or multi-locus studies (Firneno et al. 2020; Firneno and Townsend 2019). Different patterns of differentiation between mitochondrial and nuclear genomes are commonly observed when comparing relationships among species or populations in phylogeographic studies (Firneno et al. 2020; Rivera et al. 2020; Arntzen et al. 2017). Discordance can be caused by a variety of mechanisms, including differing selective pressures, sex-biased dispersal, neutral demographic processes, introgression or hybridization, incomplete lineage sorting, or a combination of mechanisms (Chiocchio et al. 2021; Dufresnes et al. 2020; Firneno et al. 2020; Ivanov et al. 2018; Thielsch et al. 2017; Toews and Brelsford 2012). Gene tree discordance has made phylogenetic reconstruction challenging and presents particular difficulties in the identification of historical demographic processes that are driving species diversity and delimitation (Degnan and Rosenberg 2009; Firneno et al. 2020; Fujita et al. 2012). The use of larger genomic datasets has helped determine the potential causes of mito-nuclear discordance, even among species with relatively large genomes (Firneno et al. 2020; Hill et al. 2019). Moreover, the use of demographic model testing and tests for phylogenetic introgression between species or populations using this type of data provide the potential to uncover the root causes of genomic incongruence (Leaché et al. 2019; Portik et al. 2017). These methods can help to identify potential biogeographic, selective, or neutral processes that might be affecting phylogenetic structure in nuclear versus mitochondrial genomes.
True toads (Bufonidae) are among the groups of organisms reported to have high levels of mito-nuclear discordance (Azevedo et al. 2003; Firneno et al. 2020; Pereyra et al. 2016; Sequeira et al. 2011). Widespread mito-nuclear discordance in toads was initially attributed to introgression of entire loci due to species hybridization, in agreement with the low selectivity of mating partners by these toads during their typically explosive breeding events (Pereyra et al. 2016; Abreu et al. 2021). However, a recent analysis using genome-wide loci found evidence of incomplete lineage sorting and not hybridization to explain discordance in toads which exhibit mito-nuclear discordance (Firneno et al. 2020). This study demonstrates the benefit of large genomic datasets when attempting to discern which mechanisms or processes may be driving patterns of among-locus gene tree heterogeneity in natural populations (Degnan and Rosenberg 2009).
Within the true toad radiation, the Rhinella granulosa species group has been hypothesized to involve high levels of hybridization and poorly resolved intraspecific relationships due to mito-nuclear incongruencies (Narvaes and Rodrigues 2009; Pereyra et al. 2016). Within this group of South American toads, 13 nominal species are currently recognized: R. granulosa, R. pygmaea, R. bergi, R. major, R. mirandaribeiroi, R. azarai, R. nattereri, R. fernandezae, R. dorbignyi, R. merianae, R. humboldti, R. bernardoi, and R. centralis (Narvaes and Rodrigues 2009). These species are distributed across much of the Neotropics, with partially overlapping ranges among species with adjacent distributions (Simon et al. 2016). Although studies incorporating multi-locus datasets have found marked genetic structure often corresponding to proposed taxon boundaries, several relationships remain poorly resolved, with conflicting patterns among loci. While these conflicts have at times been attributed to limited variability in the loci targeted or limited taxonomic sampling, some studies have proposed that poorly resolved relationships may stem from conflicting signals across markers due to hybridization between species (Pereyra et al. 2016; Simon et al. 2016). Within the clade, potential hybridization has been hypothesized to occur between the taxon pairs R. bergi and R. major, R. granulosa and R. mirandaribeiroi, and R. fernandezae and R. dorbignyi. However, there has been no formal attempt to test the hybridization scenario versus alternative gene discordance-generating mechanisms based on an adequate number of independent genomic regions.
In this contribution, we implement a phylogenomic approach to formally test the hypothesis of introgressive hybridization as a source of mito-nuclear discordance across taxa within the Rhinella granulosa species group. Based on comprehensive geographic sampling of genome-wide patterns of variation in multiple taxa across South America’s major biomes, we infer the frequency and extent of introgression based on a comparison of mitochondrial versus nuclear genetic signal, gene flow estimates and alternative models of historical demography. Based on this approach, we seek to address the following questions: Is there introgression between species within this clade, and how widespread is it? Are zones of introgression restricted to certain geographic regions, and where are they located? Lastly, how do patterns of introgression within the R. granulosa clade compare to other similarly distributed toad clades where introgression has recently been demonstrated to be rampant?
Methods
Sampling of molecular data
Our genetic sampling included 45 individuals belonging to a subset of taxa in the Rhinella granulosa species group, namely seven individuals of R. granulosa, eight R. major, 14 R. mirandaribeiroi, 13 R. merianae, one R. centralis, and two R. humbolti. As outgroups, we included four samples from the Rhinella margaritifera species group. Tissue samples were obtained from the Museum of Zoology of the University of São Paulo (MZUSP), the Amphibian and Reptile Diversity Research Center (ARDRC) at the University of Texas in Arlington, and the Louisiana State University Museum of Natural Science (LSUMNS).
We extracted genomic DNA using a phenol-chloroform extraction protocol with SeraPure SpeedBead cleanup (Sambrook and Russell 2006). Fragments of the mitochondrial 16S rRNA gene were PCR-amplified using the 16Sar and 16Sbr primers and sequenced on an ABI 3730xL sequencer following Rivera et al. (2021). We also supplemented the 16S dataset with additional sequences from R. bernardoi, R. merianae, R. mirandaribeiroi, R. pygmaea, R. major, R. bergi, R. centralis, R. dorbignyi, R. fernandezae, R. granulosa, and R. humbolti obtained from GenBank. The only species not sampled from this group for the 16S phylogeny was R. nattereri. Sequences were edited and aligned in Geneious Prime 2020.0.4 (Identification and Accession numbers in Supplementary Table S1).
We generated a double-digest restriction-site associated DNA (ddRAD) dataset following Peterson et al. (2012). Briefly, 200–500 ng of genomic DNA were digested using the SbfI (restriction site 5′‐CCTGCAGG‐3′) and MspI (restriction site 5′‐CCGG‐3′) enzymes in a single reaction using the manufacturer’s recommended buffer (New England Biolabs) for 5 h at 37 °C. Digested DNA was bead-purified before ligating barcodes and index adapters, then samples with the same index were pooled and size-selected (415–515 bp) on a Blue Pippin Prep size selector (Sage Science). The quality and concentration of final libraries were analyzed and quantified on a BioAnalyzer (Agilent) and Qubit Fluorometer 4 (Thermo Fisher Scientific). The resulting 100 bp single-end libraries were sequenced at MedGenome on an Illumina HiSeq2500.
We used the command line version of ipyrad v. 0.9.45 (Eaton and Overcast 2020) (available at https://ipyrad.readthedocs.io) to de-multiplex and assign reads to individuals based on sequence barcodes allowing no mismatches from individual barcodes, perform reference read assembly using a minimum clustering similarity threshold of 0.90, align the reads into loci, and call single nucleotide polymorphisms (SNPs). As a reference, we used the Rhinella marina genome (Edwards et al. 2018). A minimum Phred quality score (=33), sequence coverage (=6x), read length (= 35 bp), and maximum proportion of heterozygous sites per locus (=0.5) were enforced, while ensuring that variable sites had no more than two alleles (i.e., a diploid genome). Following the initial assembly, we used Matrix Condenser (de Medeiros and Farrell 2018) to assess levels of missing data across samples and then re-assembled our dataset to ensure a maximum of 25% missing data within each locus (i.e., each retained locus was sequenced in at least 25% of the sampled individuals). This strategy resulted in 45 ingroup and four outgroup samples in a matrix with less than 15% total missing data. The final dataset was composed of 16,455 SNPs over 1542 RAD loci.
Phylogenetic relationships
We inferred maximum likelihood phylogenies for both the mitochondrial dataset and a concatenated ddRAD loci dataset using IQTREE v2.1.2, utilizing the built-in model selection tool ModelFinder Plus, implementing 1000 ultrafast bootstraps only testing models of evolution and partition schemes available in MrBayes (Hoang et al. 2018; Kalyaanamoorthy et al. 2017; Minh et al. 2020). In addition to the maximum likelihood analyses, we performed phylogenetic inference for both the mitochondrial dataset and a concatenated ddRAD loci dataset under a Bayesian framework using MrBayes 3.2.6 (Ronquist et al. 2012), implementing three independent runs of four Markov chains of 10 million generations each and sampling every 1000 generations with the first 25% generations discarded as burn-in. We used the same best-fit model found by ModelFinder Plus in the Bayesian analyses. We used Tracer 1.7 (Rambaut et al. 2018) to assess whether Markov chain mixing was adequate (effective sample sizes >200) and to visually assess model parameter stationarity and convergence between runs. We then summarized a 50% majority-rule consensus tree and used iTol to edit and visualize trees (Letunic and Bork 2019).
We also compared the nuclear IQTREE results to a species-tree inferred under a multispecies coalescent framework utilizing the nuclear data in SVD Quartets (Chifman and Kubatko 2014) as implemented in the command line version of PAUP v. 4 (Swofford 2002). In this analysis, we sampled all possible quartets and estimated node support based on 1000 bootstrap replicates.
Population genetic structure
To determine populations and admixture in the R. granulosa species group, we filtered SNPs as described above but excluding outgroup samples using ipyrad and VCFtools (Danecek et al. 2011), resulting in a dataset composed of 20,527 SNPs. SNPs were filtered further to only include one random SNP per RAD locus, resulting in a final dataset of 1527 unlinked SNPs. We then determined the best-fit number of genetic clusters (K) using the maximum likelihood method ADMIXTURE with 20 replicates per K and a 10-fold crossvalidation to determine the best-fit K (Alexander et al. 2009). Specifically, the best-fit K was determined as the replicate with the lowest cross-validation error. In addition to our ADMIXTURE analysis, we used TESS3r in R, running 50 replicates per K, to both assess genomic admixture as well as estimate and visualize spatially informed population structure in geographic space (Caye et al. 2016, 2018).
To further characterize population structure, we used the non-parametric method of discriminant analysis of principal components (DAPC), implemented in the R package adegenet (Jombart and Ahmed 2011; Jombart et al. 2010). The find.clusters function was used to test the fit of 1–20 clusters (K). The K with the lowest Bayesian information criterion (BIC) score was considered the best-fit number of clusters.
The resulting ancestry coefficient matrices (Q-matrices) from the ADMIXTURE analysis were then imported into QGIS (QGIS Geographic Information System. QGIS Association. http://www.qgis.org) to allow visualizing patterns of genetic clustering and admixture in geographic space.
Gene flow
To infer potential hybridization or gene flow among taxa, we calculated Patterson’s D statistic and the related admixture fraction estimates, or f4-ratio statistics, using the program Dsuite (Patterson et al. 2012; Malinsky et al. 2020). This approach, also known as an ABBA-BABA test, uses a four-taxon fixed phylogeny—in the form (((P1,P2)P3)O)—to quantify the proportion of shared alleles that can be attributed to horizontal transfer among the populations considered. This approach assumes that a typical ancestral (“A”) and derived (“B”) allele pattern among four terminal taxa should generate a BBAA structure. Under incomplete lineage sorting, conflicting ABBA and BABA patterns should occur in equal frequencies, resulting in a D statistic = 0. If, however, introgression between P3 and P1 or P2 has occurred, there should be an excess of one of these two patterns, and thus a D statistic significantly different from 0 (Durand et al. 2011; Patterson et al. 2012). We used the f‐branch or fb(C) metric to tease apart potentially correlated f4‐ratio statistics and estimate gene flow events between internal branches on the phylogeny (Martin et al. 2013; Malinsky et al. 2018). Dsuite uses a VCF file and a jackknifing approach to assess correlations in allele frequencies between closely related species (Malinsky et al. 2020). Within Dsuite, we used the Dtrios and Fbranch programs to identify introgression between all combinations of species, using R. margaritifera as the outgroup taxa, and applied the Benjamini-Hochberg (BH) correction to control for false discovery rate using a p value of 0.05.
Demographic modeling
We use the full-likelihood, multispecies coalescent method Generalized Phylogenetic Coalescent Sampler (G-PhoCS) v.1.3.2 on the CIPRES Science Gateway to estimate demographic parameters such as effective population sizes and divergence times along the evolutionary history of the Rhinella granulosa species group (Miller et al. 2010; Gronau et al. 2011). For this analysis, we used the phylogenetic topology inferred by our maximum likelihood and Bayesian analyses on the ddRAD data (see Results). To reduce computing time, we used a maximum of 10 randomly chosen individuals per delimited genetic cluster and re-ran this dataset through the filtering pipeline resulting in 2744 unlinked SNPs. G-PhoCS ran an automatic fine-tuning for 500 steps, running the entire analysis twice independently for 500,000 generations, then again for one million generations, sampling every 1000 generations. We then merged runs using LogCombiner and discarded the first 10% of samples as burn-in (Pramuk et al. 2007; Drummond and Rambaut 2007; Alonso et al. 2012). The Dsuite analysis determined no significant gene flow between populations (see Results), so we did not apply migration bands in GPhoCS analyses. We followed Prates et al. (2018) to select distributions for the priors of the θ and τ parameters (scripts available at https://github.com/ivanprates/2018_Anolis_EcolEvol), applying a gamma distribution with parameters α = 2.0 and β = 30. We used TRACER 1.7 to assess proper chain mixing and convergence based on the log output files (Rambaut et al. 2018). We then converted θ estimates to Ne, in number of individuals, using the relationship θ = 4Neμ, and τ estimates to T, in years, using the relationship τ = Tμ/g. To apply these conversions, we used a nuclear mutation rate (μ) of 2.4 × 109 (Prates et al. 2016, 2018) and a generation time (g) of 2 years for bufonid toads (Lever 2001).
Results
Phylogenetic relationships
Phylogenetic analyses of the 16S mitochondrial gene based on both maximum likelihood and Bayesian approaches yielded nearly identical relationships (Fig. 1). Overall, most species-level taxa currently recognized within the R. granulosa species group were inferred as monophyletic. Mitochondrial analyses inferred 10 major clades, most of which corresponded to currently recognized taxa. Moreover, most of these clades were geographically coherent, with samples assigned to the same taxon generally clustering in geographic space, as follows: R. dorbignyi and R. fernandezae in the southern Pampas; R. major across southern and eastern Amazonia; R. granulosa in the Caatinga and northern Atlantic Forest; R. centralis in the northern Andes, R. humbolti in northern Amazonia; R. meriane in central Amazonia; and R. mirandaribeiroi across the Cerrado (Locality map characterized by 16S data is available in Supplementary Fig. S1).
The Bayesian mitochondrial analysis resulted in a phylogeny with higher overall relative support for most of the internal nodes (PP = 0.90–1.0) that correspond to relationships between species-level taxa. The only relationship that received lower support (PP = 0.64) was the position of R. mirandaribeiroi and the clade composed of R. granulosa, R. centralis, R. humbolti, and R. meriane. (Fig. 1). This phylogeny placed R. major as the sister to all other remaining taxa within this clade; within this clade, R. pygmaea, R. azarai and R. bergi formed their own respective clades, and R. dorbingyi and R. fernandezae formed a subclade. The other major clade within the species group included R. mirandaribeiroi, R. granulosa, R. centralis, R. humbolti, and R. merianae, nested in this order relative to the root.
Phylogenetic relationships based on the ddRADseq dataset—which included only a subset of the taxa represented in the mitochondrial trees—were overall highly supported (Figs. 1 and 2A). Similar to the mitochondrial analyses, all of the currently recognized species were inferred to be monophyletic and geographically coherent, except for one R. granulosa individual, which was inferred as nested within the R. mirandaribeiroi clade (Figs. 1 and 2A). The ddRAD phylogeny included two clades: one composed of R. granulosa and R. mirandaribeiroi, and another composed of R. major, R. merianae, R. humbolti, and R. centralis (Figs. 1 and 2A). Nuclear phylogenetic analyses under both the concatenated (IQTREE, MrBayes; Fig. 1) and multispecies coalescent (SVD Quartets; Supplementary Fig. S2) approaches yielded the same species topology.
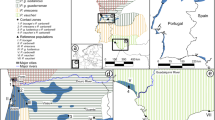
A Maximum likelihood/Bayesian phylogeny incorporating data from GPhoCS for R. granulosa clade focal species using ddRADseq data, and corresponding ADMIXTURE, DAPC, and TESS3r plots. Branch widths are scaled to estimated population sizes (G-PhoCS) and asterisks represent BS/PP > 95/0.99. B DAPC plots for K = 4–6, C TESS3r plots for K = 4–6.
While the sample composition of clades was similar between mitochondrial and nuclear analyses, the relationships between taxon clades differed between marker types. Most notably, the affinity between R. major and the clade composed of R. centralis, R. humbolti, and R. merianae varied by marker type. Rhinella major was sister to all other species in the 16S phylogeny, while in the nuclear phylogeny it was inferred to form a clade with R. centralis, R. humbolti, and R. merianae (Fig. 1). Additionally, the placement of R. mirandaribeiroi in relation to R. granulosa differed between mitochondrial and nuclear markers in the 16S phylogeny. R. mirandaribeiroi was sister to a clade formed by R. granulosa, R. centralis, R. humbolti, and R. merianae, while in the nuclear phylogeny, R. mirandaribeiroi shared a clade with R. granulosa only (Fig. 1).
Population structure
ADMIXTURE results indicated the K value with the lowest crossvalidation error was four, which was a cluster formed by samples assigned to R. major (orange), one R. granulosa cluster (blue), one R. mirandaribeiroi cluster (purple), and a cluster combining samples assigned to R. merianae, R. humbolti, and R. centralis (yellow; Fig. 2A). The only individuals that were found to be admixed had alleles that traced back to the R. granulosa and the R. mirandaribeiroi clusters (Fig. 2A). The DAPC analysis yielded similar BIC scores for K = 4–6 (Supplementary Fig. S3). K = 4 resulted in the same clusters identified by ADMIXTURE, while K = 5 split the ADMIXTURE cluster composed of R. merianae, R. humbolti, and R. centralis into two clusters, one composed of R. merianae samples and the other of R. humbolti and R. centralis samples (Fig. 2). K = 6 further subdivided R. mirandaribeiroi into two clusters, one of which included the R. granulosa individual inferred to be nested within the R. mirandaribeiroi cluster (Fig. 2A, B). ADMIXTURE results for K = 5–6 yielded the same clusters as the results from the DAPC analysis (Supplementary Fig. S3; cross-validation plots: Supplementary Fig. S4). TESS3r results were very similar to both ADMIXTURE and DAPC results (Fig. 2A, B). Overall, there was slight amounts of admixture among R. mirandaribeiroi, R. granulosa, R. humbolti, and R. centralis for K = 4–6 (Fig. 2A).
Similar to the phylogenetic analyses, major genetic clusters inferred by ADMIXTURE were geographically coherent, each restricted to a certain portion of South America. Specifically, the cluster composed of samples assigned to R. major was restricted to the Amazon region; the cluster composed of R. merianae, R. humbolti, and R. centralis samples was distributed across the Amazon and the Northern Andes; the cluster composed of samples assigned to R. granulosa was distributed across the Northern Atlantic Forest in eastern Brazil; and the cluster composed of samples assigned to R. mirandaribeiroi was distributed across the Cerrado in central Brazil (Fig. 3). The spatially explicit TESS3r resulting maps showing the diffusion of ancestry coefficients across geographic space shows genetic groups are geographically clustered for K = 4–6 (Fig. 2C).
D-statistics
Analyses of introgression based on the ddRAD data using Dsuite resulted in only one trio ((R. mirandaribeiroi, R. granulosa) R. major) out of 20 trios tested that had a significant D-statistic (0.41; Supplementary Table S2), pointing to a larger proportion of shared alleles between R. granulosa and R. major than expected based solely on incomplete lineage sorting. However, after applying the Benjamini-Hochberg (BH) correction to control for the false discovery rate, none of the trios tested were significant (Supplementary Table S2). These results were corroborated using the fb(C) metric analysis, which showed an fb statistic of 0.15 between R. granulosa and R. major, but it was not significant (Fig. 4 and Supplementary Table S3). Taken together, these results support that introgression is not an important factor behind shared allele patterns among any of the groups considered.
Demographic inference
The G-PhoCS analysis estimated the Rhinella granulosa species group to date back to about 5 million years before present (mybp) (mean value; 95% highest posterior density (HPD) = 4.817–5.092; Fig. 2 and Supplementary Table S4). Sister species divergences within the species group dated back to the mid-Pleistocene (Fig. 2 and Supplementary Table S4). The divergence between R. granulosa and R. mirandaribeiroi was estimated to date back to 1.026 mybp (HPD = 0.983–1.075), while the divergence between R. humbolti and R. centralis dated back to 1.496 mybp (HPD = 1.258–1.733). In turn, the most recent common ancestor of R. merianae, R. humbolti, and R. centralis dated back to 2.263 mybp (2.158–2.358 95% HPD), while the ancestor of these three species and R. major dated back to 4.826 mybp (HPD = 4.625–4.983 95% HPD). The effective population size estimates resulted in a root population size of ~1.3 million, remaining stable along the R. granulosa-mirandaribeiroi branch. This lineage massively expanded to Amazonia (along the R. major-merianae-humbolti-centralis branch), then experienced a bottleneck after R. major diverged, and then expanded again after R. merianae diverged (Fig. 2 and Supplementary Table S4). Estimates of current population sizes for each species ranged from ~145,833–1,093,750 (Supplementary Table S4).
Discussion
Based on range-wide geographic sampling of mitochondrial and nuclear genetic variation in a widespread species group of neotropical toads, we found only partial concordance between currently recognized species-level taxon boundaries and patterns of genetic structure. While most taxa within the R. granulosa species group corresponded to clades, genetic clustering analyses sometimes grouped distinct taxonomic units into a single cluster. Moreover, levels of admixture between inferred clusters were limited and restricted to a single sister taxon pair (R. granulosa and R. mirandaribeiroi), while D-statistics estimation provided no support for the hypothesis that allele sharing between non-sister taxa is a result of horizontal transfer. These findings contradict previous assertions of widespread cryptic diversity and of gene flow and introgression across taxa in the R. granulosa clade, a proposed scenario based on small numbers of loci (Guerra et al. 2011; Pereyra et al. 2016). Lastly, our analyses suggest that diversification events within the Rhinella granulosa clade mostly dated back to the early Pliocene, which is later than species divergences in other closely related clades, as observed in the closely-related R. marina species group (Rivera et al. 2022).
Previous analyses of phylogenetic relationships in the R. granulosa clade based on multi-locus datasets have resulted in unresolved interspecific relationships and evidence of mito-nuclear discordance (Azevedo et al. 2003; Sequeira et al. 2011; Pereyra et al. 2016). By incorporating thousands of ddRADseq nuclear loci, however, we were able to improve phylogenetic resolution within this clade (Fig. 2). Previous studies have, in several cases, inferred relationships similar to ours. Specifically, both our study and that of Pereyra et al. (2016) found a sister relationship between R. granulosa and R. mirandaribeiroi, as well as phylogenetic clustering of R. centralis, R. humbolti, and R. merianae. However, while Pereyra et al. (2016) found R. major to be the sister of all other species, our analysis inferred R. major as sister to the clade composed of R. centralis, R. humbolti, and R. merianae with high support (Fig. 2). These discrepancies between studies could have originated from differences in the genetic markers used. For instance, although we did not observe a clear signal of mitochondrial introgression across taxa based on 16S, an analysis by Pereyra et al. (2016) incorporating other mitochondrial genes found several taxa to be nonmonophyletic. This is the case, for instance, of R. bergi, R. major, and R. fernandezae, which were thus hypothesized to experience past introgressive events. By assessing the mitochondrial DNA in this study separate from the thousands of nuclear markers generated, our analysis of genetic structure and introgression based on extensive nuclear data found no support for the idea that hybridization played a major role in the evolutionary history of these toads.
Besides hybridization, another mechanism that may generate a pattern of allele sharing among non-sister taxa is incomplete lineage sorting, as reported in studies of several other clades, including birds, lizards, mammals, plants, and flies (Jakob and Blattner 2006; Pollard et al. 2006; McGuire et al. 2007; McKay and Zink 2010; Wang et al. 2018). Our analyses of the R. granulosa group are consistent with the idea that instances of mito-nuclear discordance can be attributed to incomplete lineage sorting. For instance, R. granulosa and the genetic group formed by the samples originally assigned to R. centralis, R. humbolti, and R. meriane were inferred as sister clades in the mitochondrial phylogeny but not in the nuclear one. The D-statistics estimates supported that the observed proportion of shared nuclear alleles among these two groups is no different from that expected under incomplete lineage sorting. Incomplete lineage sorting has been similarly invoked to explain similar patterns of genealogical heterogeneity in phylogenetic studies across the tree of life (Liu et al. 2009; Blanco-Pastor et al. 2012; Doronina et al. 2015). Given our sampling of the species group, a full sampling of all ~13 species should be used to more robustly assess gene flow and demographic change among species in a hypothesis-testing framework.
Alternatively, a pattern of taxon paraphyly in gene genealogies can result from purifying selection, which reduces genetic diversity at linked sites, such as the different genes that comprise the mitochondrial genome (Cvijović et al. 2018). Accordingly, studies have found signatures of selection on the mitochondrial genes of species that show patterns of mito-nuclear discordance (Morales et al. 2015). It is currently unclear whether selection may have contributed to a pattern of paraphyletic taxa as inferred by mitochondrial genes in the R. granulosa species group (Pereyra et al. 2016). Nevertheless, our analyses of thousands of nuclear loci suggest that shared alleles across non-sister taxa conform to expectations under incomplete lineage sorting and not introgressive hybridization. As such, our analyses challenge previous assertions of rampant hybridization in the evolutionary history of the R. granulosa species group, similar to recent proposals for other vertebrate clades (Wang et al. 2018). We note that our taxon sampling was more limited than that of previous studies of this clade due to challenges in accessing samples from throughout its vast distribution. Additionally, the relatively short 16S fragment sequenced in this study (564 base pairs) precludes accurate tests of selection among this group. It is possible that inclusion of additional taxa, as well as better sampling of each taxon across their respective ranges, especially near any potential contact zones, could reveal more complex patterns of admixture.
Our finding of negligible introgression across taxa in the R. granulosa species group contrasts with patterns observed in other closely related toad clades. For instance, the Rhinella marina species group experienced many instances of genetic admixture among species over its evolutionary history. Recent investigation based on thousands of genome-wide loci inferred multiple instances of admixture across and within taxa in the R. marina group, as well as past and ongoing pulses of gene flow (Rivera et al. 2022). Why we observe such distinct patterns of introgression across taxa in two closely related toad clades is unclear, especially considering the R. marina group is similarly diverse, though this particular study had larger sample sizes in comparison. Interestingly, higher levels of introgression do not seem to be associated with recent population divergences, a pattern suggested for other species groups (Singhal and Moritz 2013; Singhal and Bi 2017). Instead, estimates for the R. marina clade suggest older divergences overall than those in the R. granulosa clade (Rivera et al. 2022).
Instead, it is possible that these contrasting patterns across clades reflect differences in organismal attributes. Toads are generally reported to be prone to low selectivity of mates during their typically explosive breeding events, which also seems to apply to the R. granulosa species group; for instance, interspecific amplexus between co-distributed Rhinella granulosa and other species from the Rhinella and Ceratophrys genera have been reported (Abreu et al. 2021; Gama et al. 2020). Nevertheless, our findings could suggest that these apparently permeable prezygotic reproductive barriers do not necessarily lead to gene transfer across interbreeding species. If that is the case, it is possible that species in the R. granulosa species group have developed post-zygotic barriers to hybridization that species in other closely related clades have not, as appears to be the case of the R. marina species group, though more evidence is required to test this assertion (Pereyra et al. 2016; Bonnet et al. 2017; Abreu et al. 2021). Future studies of genetic structure across clades will benefit from incorporating information on species’ reproductive biology and natural history. Unfortunately, this much-needed information is largely lacking for species-rich clades of tropical organisms, as is the case of the intriguing true toads.
Data availability
Sequence data are available on GenBank (ON692445- ON692509) and demultiplexed sequence files are available on the Sequence Read Archive (PRJNA858546). Associated scripts and input files have been made available on dryad: https://doi.org/10.5061/dryad.p2ngf1vtc.
References
Abreu LM, Santos JEM, Corrêa AT, Ribeiro MV (2021) Interspecific amplexus between two sympatric species, Rhinella granulosa (Spix, 1824) and Rhinella crucifer (Wied-Neuwied, 1821) (Anura: Bufonidae) in Ilhéus, Bahia, Brazil. HNO 14:869–871
Alexander DH, Novembre J, Lange K (2009) Fast model-based estimation of ancestry in unrelated individuals. Genome Res 19:1655–1664
Alonso R, Crawford AJ, Bermingham E (2012) Molecular phylogeny of an endemic radiation of Cuban toads (Bufonidae: Peltophryne) based on mitochondrial and nuclear genes: origin and diversification of Cuban toads. J Biogeogr 39:434–451
Arntzen JW, de Vries W, Canestrelli D, Martínez-Solano I (2017) Hybrid zone formation and contrasting outcomes of secondary contact over transects in common toads. Mol Ecol 26:5663–5675
Azevedo MFC, Foresti F, Ramos PRR, Jim J (2003) Comparative cytogenetic studies of Bufo ictericus, B. paracnemis (Amphibia, Anura) and an intermediate form in sympatry. Genet Mol Biol 26:289–294
Blanco-Pastor JL, Vargas P, Pfeil BE (2012) Coalescent simulations reveal hybridization and incomplete lineage sorting in Mediterranean Linaria. PLoS ONE 7:e39089
Bonnet T, Leblois R, Rousset F, Crochet P-A (2017) A reassessment of explanations for discordant introgressions of mitochondrial and nuclear genomes. Evolution 71:2140–2158
Caye K, Jay F, Michel O, Francois O (2018) Fast inference of individual admixture coefficients using geographic data. Ann Appl Stat 12:586–608
Caye K, Deist T, Martins H, Michel O, Francois O (2016) TESS3: fast inference of spatial population structure and genome scans for selection. Mol Ecol Resour 16:540–548
Chifman J, Kubatko L (2014) Quartet inference from SNP data under the coalescent model. Bioinformatics 30:3317–3324
Chiocchio A, Arntzen JW, Martínez-Solano I et al. (2021) Reconstructing hotspots of genetic diversity from glacial refugia and subsequent dispersal in Italian common toads (Bufo bufo). Sci Rep. 11:260
Cvijović I, Good BH, Desai MM (2018) The effect of strong purifying selection on genetic diversity. Genetics 209:1235–1278
Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA et al. (2011) The variant call format and VCFtools. Bioinformatics 27:2156–2158
Degnan JH, Rosenberg NA (2009) Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends Ecol Evol 24:332–340
Doronina L, Churakov G, Shi J, Brosius J, Baertsch R, Clawson H et al. (2015) Exploring massive incomplete lineage sorting in Arctoids (Laurasiatheria, Carnivora). Mol Biol Evol 32:3194–3204
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214
Dufresnes C, Nicieza AG, Litvinchuk SN, Rodrigues N, Jeffries DL, Vences M et al. (2020) Are glacial refugia hotspots of speciation and cytonuclear discordances? Answers from the genomic phylogeography of Spanish common frogs. Mol Ecol 29:986–1000
Durand EY, Patterson N, Reich D, Slatkin M (2011) Testing for ancient admixture between closely related populations. Mol Biol Evol 28:2239–2252
Eaton DAR, Overcast I (2020) ipyrad: Interactive assembly and analysis of RADseq datasets. Bioinformatics 36:2592–2594
Edwards RJ, Tuipulotu DE, Amos TG, O’Meally D, Richardson MF, Russell TL et al. (2018) Draft genome assembly of the invasive cane toad, Rhinella marina. GigaScience 7:1–13
Firneno Jr TJ, Townsend JH (2019) Evaluation of species boundaries in sympatric and parapatric populations of Mesoamerican toads. Zool Scr 48:454–465
Firneno Jr TJ, O’Neill JR, Portik DM, Emery AH, Townsend JH, Fujita MK (2020) Finding complexity in complexes: assessing the causes of mitonuclear discordance in a problematic species complex of Mesoamerican toads. Mol Ecol 29:3543–3559
Fujita MK, Leaché AD, Burbrink FT, McGuire JA, Moritz C (2012) Coalescentbased species delimitation in an integrative taxonomy. Trends Ecol Evol 27:480–488
Gama V, dos Santos Protázio A, dos Santos, Protázio A (2020) Interspecific amplexus between male Rhinella granulosa (Spix, 1824) and a metamorph of Ceratophrys joazeirensis Mercadal, 1986 (Amphibia: Anura) in a temporary pond in the Caatinga, Bahia State, Northeastern Brazil. Herpetol Notes 13:749–751
Gronau I, Hubisz MJ, Gulko B, Danko CG, Siepel A (2011) Bayesian inference of ancient human demography from individual genome sequences. Nat Genet 43:1031–1034
Guerra C, Baldo D, Rosset S, Borteiro C, Kolenc F (2011) Advertisement and release calls in Neotropical toads of the Rhinella granulosa group and evidence of natural hybridization between R. bergi and R. major (Anura: Bufonidae). Zootaxa 3092:26–42
Hill GE, Havird JC, Sloan DB, Burton RS, Greening C, Dowling DK (2019) Assessing the fitness consequences of mitonuclear interactions in natural populations. Biol Rev Camb Philos Soc 94:1089–1104
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522
Ivanov V, Lee KM, Mutanen M (2018) Mitonuclear discordance in wolf spiders: genomic evidence for species integrity and introgression. Mol Ecol 27:1681–1695
Jakob SS, Blattner FR (2006) A chloroplast genealogy of Hordeum (Poaceae): long-term persisting haplotypes, incomplete lineage sorting, regional extinction, and the consequences for phylogenetic inference. Mol Biol Evol 23:1602–1612
Jombart T, Ahmed I (2011) adegenet 1.3-1: new tools for the analysis of genomewide SNP data. Bioinformatics 27:3070–3071
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet 11:94
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589
Leaché AD, Portik DM, Rivera D, Rödel MO, Gvoždík V, Penner J et al. (2019) Exploring rainforest diversification using demographic model testing in the African foam-nest treefrog (Chiromantis rufescens). J Biogeogr 46:2706–2721
Letunic I, Bork P (2019) Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47:W256–W259
Lever C (2001) The cane toad: the history and ecology of a successful colonist. Westbury Academic and Scientific Publishing, Otley, Yorkshire, UK
Liu L, Yu L, Kubatko L, Pearl DK, Edwards SV (2009) Coalescent methods for estimating phylogenetic trees. Mol Phylogenet Evol 53:320–328
Malinsky M, Matschiner M, Svardal H (2020) Dsuite—Fast D‐statistics and related admixture evidence from VCF files. Mol Ecol Resour 21:584–595
Malinsky M, Svardal H, Tyers AM, Miska EA, Genner MJ, Turner GF et al. (2018) Whole-genome sequences of Malawi cichlids reveal multiple radiations interconnected by gene flow. Nat Ecol Evol 2:1940–1955
Martin SH, Dasmahapatra KK, Nadeau NJ, Salazar C, Walters JR, Simpson F et al. (2013) Genome-wide evidence for speciation with gene flow in Heliconius butterflies. Genome Res 23:1817–1828
McGuire JA, Linkem CW, Koo MS, Hutchison DW, Lappin AK, Orange DI et al. (2007) Mitochondrial introgression and incomplete lineage sorting through space and time: phylogenetics of crotaphytid lizards. Evolution 61:2879–2897
McKay BD, Zink RM (2010) The causes of mitochondrial DNA gene tree paraphyly in birds. Mol Phylogenet Evol 54:647–650
de Medeiros BAS, Farrell BD (2018) Whole-genome amplification in doubledigest RADseq results in adequate libraries but fewer sequenced loci. PeerJ 6:e5089
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 2010 Gateway Computing Environments Workshop (GCE), p 1–8
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A et al. (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol 37:1530–1534
Morales HE, Pavlova A, Joseph L, Sunnucks P (2015) Positive and purifying selection in mitochondrial genomes of a bird with mitonuclear discordance. Mol Ecol 24:2820–2837
Narvaes P, Rodrigues MT (2009) Taxonomic revision of Rhinella granulosa species group (Amphibia, Anura, Bufonidae), with a description of a new species. Arquivos De Zoologia 40:1–73
Patterson N, Moorjani P, Luo Y, Mallick S, Rohland N, Zhan Y et al. (2012) Ancient admixture in human history. Genetics 192:1065–1093
Pereyra MO, Baldo D, Blotto BL, Iglesias PP, Thomé MTC, Haddad CFB et al. (2016) Phylogenetic relationships of toads of the Rhinella granulosa group (Anura: Bufonidae): a molecular perspective with comments on hybridization and introgression. Cladistics 32:36–53
Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE (2012) Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 7:e37135
Pollard DA, Iyer VN, Moses AM, Eisen MB (2006) Widespread discordance of gene trees with species tree in Drosophila: evidence for incomplete lineage sorting. PLoS Genet 2:e173
Portik DM, Leaché AD, Rivera D, Barej MF, Burger M, Hirschfeld M et al. (2017) Evaluating mechanisms of diversification in a Guineo-Congolian forest frog using demographic model selection. Mol Ecol 26:5245–5263
Pramuk JB, Robertson T, Sites JW, Noonan BP (2007) Around the world in 10 million years: biogeography of the nearly cosmopolitan true toads (Anura: Bufonidae). Glob Ecol Biogeogr 17:72–83
Prates I, Rivera D, Rodrigues MT, Carnaval AC (2016) A mid-Pleistocene rainforest corridor enabled synchronous invasions of the Atlantic Forest by Amazonian anole lizards. Mol Ecol 25:5174–5186
Prates I, Penna A, Rodrigues MT, Carnaval AC (2018) Local adaptation in mainland anole lizards: Integrating population history and genome-environment associations. Ecol Evol 8:11932–11944
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67:901–904
Rivera D, Prates I, Rodrigues MT, Carnaval AC (2020) Effects of climate and geography on spatial patterns of genetic structure in tropical skinks. Mol Phylogenet Evol 143:106661
Rivera D, Prates I, Firneno Jr. TJ, Rodrigues MT, Caldwell JP, Fujita MK (2022) Phylogenomics, introgression, and demographic history of South American true toads (Rhinella). Mol Ecol 31:978–992
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S et al. (2012) MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Syst Biol 61:539–542
Sambrook J, Russell DW (2006) Purification of nucleic acids by extraction with phenol:chloroform. Cold Spring Harb Protoc. https://doi.org/10.1101/pdb.prot4455.
Sequeira F, Sodré D, Ferrand N, Bernardi JAR, Sampaio I, Schneider H et al. (2011) Hybridization and massive mtDNA unidirectional introgression between the closely related Neotropical toads Rhinella marina and R. schneideri inferred from mtDNA and nuclear markers. BMC Evol Biol 11:264
Simon MN, Machado FA, Marroig G (2016) High evolutionary constraints limited adaptive responses to past climate changes in toad skulls. Proc Biol Sci 283:20161783
Singhal S, Moritz C (2013) Reproductive isolation between phylogeographic lineages scales with divergence. Proc Biol Sci 280:20132246
Singhal S, Bi K (2017) History cleans up messes: the impact of time in driving divergence and introgression in a tropical suture zone. Evolution 71:1888–1899
Swofford DL (2002) PAUP: phylogenetic analysis using parsimony (and other methods), Version 4.0 Beta 10. Sinauer Associates, Sunderland
Thielsch A, Knell A, Mohammadyari A, Petrusek A, Schwenk K (2017) Divergent clades or cryptic species? Mito-nuclear discordance in a Daphnia species complex. BMC Evol Biol 17:227
Toews DPL, Brelsford A (2012) The biogeography of mitochondrial and nuclear discordance in animals. Mol Ecol 21:3907–3930
Wang K, Lenstra JA, Liu L, Hu Q, Ma T, Qiu Q et al. (2018) Incomplete lineage sorting rather than hybridization explains the inconsistent phylogeny of the wisent. Commun Biol 1:169
Acknowledgements
We thank all the Brazilian collectors who made this study possible and particularly students in MTR’s laboratory for field collection and support. Brazil’s Instituto Chico Mendes de Conservação da Biodiversidade issued collecting permits (SISBIO 36753-1, 36753-4, and 27290-3). This work was co-funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; BIOTA 2013/50297-0), the National Science Foundation (DEB 1343578), and the National Aeronautics and Space Administration through the Dimensions of Biodiversity Program. JPC was funded by the National Science Foundation (DEB 9200779 and DEB 9505518). MTR acknowledges additional funding from FAPESP grants 2003/10335-8, 2011/50146-6, 2011/50206‐9, 2012/15754-8, and 2017/08357-6. DR was funded by NSF GRFP. IP was funded by NSF grant DEB 1754398.
Author information
Authors and Affiliations
Contributions
Contributions DR, IP, and MKF discussed and designed the study. IP provided expertise for many analyses. DR compiled the dataset, ran the analyses, and wrote the first draft and revised versions of the manuscript. IP provided significant input to the first draft and revised versions of the manuscript. JPC, MTR, and MKF contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Associate editor: Ben Evans.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rivera, D., Prates, I., Caldwell, J.P. et al. Testing assertions of widespread introgressive hybridization in a clade of neotropical toads with low mate selectivity (Rhinella granulosa species group). Heredity 130, 14–21 (2023). https://doi.org/10.1038/s41437-022-00571-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41437-022-00571-9