Abstract
Recently, deleterious effects of aldosterone on the kidney via mineralocorticoid receptors (MRs) have been noted. MR antagonists have been reported to show significant antialbuminuric effects when added to angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers. However, a decrease in the estimated glomerular filtration rate (eGFR) has been reported during MR antagonist treatment. On the other hand, although the eGFR often decreases, significant reductions in total mortality and cardiovascular events have been observed in large-scale clinical trials in patients with chronic heart failure. What are the implications of the changes in eGFR due to MR antagonist treatment? Glomerular hyperfiltration has been reported to occur with an aldosterone excess, and it can be seen that relative glomerular hyperfiltration is rapidly corrected with MR antagonism, even without aldosterone excess. This is reflected in the initial temporary decrease in the eGFR. After MR antagonist treatment, eGFR decreases temporarily, and it appears that renal function has deteriorated. However, if renal function has actually deteriorated, a reduction in all-cause and cardiovascular death is unlikely to occur in the clinical studies in patients with chronic heart failure. That is, the initial transient decrease in eGFR by the MR antagonist appears to work effectively to provide fine adjustment of glomerular pressure, and this approach works advantageously to suppress long-term cardiovascular events. It is expected that a number of long-term, large-scale clinical research trials targeting renal events and all-cause and cardiovascular death in CKD patients treated with an MR antagonist will be planned.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) affects a large and growing number of patients, and the control of its onset and progression has become an important clinical issue worldwide [1]. CKD is a risk factor for end-stage renal failure as well as a major risk factor for the development of cardiovascular diseases, and it affects life expectancy [2,3,4,5]. The therapeutic goals are to suppress the decrease in eGFR and reduce proteinuria and albuminuria. However, a common clinical experience during the treatment of CKD is that these two goals cannot both be met. After antihypertensive drugs are started, there is a decrease in eGFR, whereas proteinuria and albuminuria are also decreasing at that time.
Recently, deleterious effects of aldosterone on the kidney via mineralocorticoid receptors (MRs) have been noted [6, 7]. MR antagonists have been reported to show significant antiproteinuric and antialbuminuric effects when added to angiotensin-converting enzyme (ACE) inhibitor or angiotensin II type 1 receptor blocker (ARB) therapy [8,9,10,11,12,13]. However, a decrease in eGFR has been reported during MR antagonist treatment. On the other hand, although eGFR is frequently decreased, significant reductions in total mortality and cardiovascular events by MR antagonists have been observed in large-scale clinical trials in patients with chronic heart failure [14,15,16]. What are the implications of the changes in eGFR due to MR antagonist treatment? In this review paper, the renal effects of MR antagonist treatment are summarized in terms of the effect on eGFR.
An early temporary decrease in eGFR during treatment and a decrease in eGFR over the years have different meanings
Decreased eGFR is an independent risk factor for all-cause mortality and death from cardiovascular disease [17]. This is particularly evident when observing changes in eGFR on a yearly basis, which is a significant risk factor for overall mortality and coronary artery disease [5, 18,19,20]. There were many renal and cardiovascular events in patients with decreases of at least 12.7% in renal function after treatment with an ACE inhibitor or ARB in the ONTARGET (Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial) trial or the TRANSCEND (Telmisartan Randomized Assessment Study in ACE Intolerant Participants with Cardiovascular Disease) trial [21].
On the other hand, after antihypertensive drugs are started, a temporary, rapid decline in eGFR may be observed. In this case, eGFR often recovers to some extent early thereafter. From the analysis of the RENAAL (Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus with the Angiotensin II antagonist Losartan) trial, Holtkamp et al. showed that the ARB-treated group had a significant decrease in eGFR compared with the placebo group at 3 months after the start of treatment. However, long-term changes in eGFR were slower in the ARB-treated group [22]. Such a temporary decrease in eGFR after the start of antihypertensive drugs is considered to restore glomerular hyperfiltration [23]. On discontinuation of ACE inhibitor or ARB therapy, eGFR recovers [24], indicating that it does not cause irreversible renal dysfunction [25]. The degree of initial eGFR decline correlated inversely with the subsequent decline in eGFR [23]. At the time of strict blood pressure control, an early transient decrease in eGFR is also often observed [26]. However, if blood pressure is properly controlled, this initial transient eGFR decline does not appear to be harmful [27,28,29].
In CKD and hypertension treatment, the implications are different between an early temporary decrease in eGFR after the start of treatment and the decrease in eGFR lasting for a relatively long time. In the case of the early, temporary decrease, eGFR mostly falls within a certain range, it does not decrease greatly, and it recovers, although it is slightly lower than the previous value, and there is no continuous decline thereafter. In the long run, it is likely to be beneficial. On the other hand, in the long-term decrease case, it is an obvious risk factor for overall mortality and an independent risk factor for cardiovascular events.
Implications of a transient, rapid eGFR decline after initiation of treatment as discussed from clinical studies using a sodium–glucose cotransporter 2 (SGLT-2) inhibitor
A significant reduction in the primary endpoint consisting of the composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke was shown in the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial using the SGLT-2 inhibitor empagliflozin [30]. In its subanalysis, empagliflozin suppressed the transition to overt proteinuria and renal events. The change in eGFR with empagliflozin was very similar to that with ACE inhibitor or ARB therapy. It dropped temporarily by approximately 4 weeks and remained stable at a level lower than the previous value through 12 weeks. At 192 weeks, eGFR was significantly higher than in the placebo group [31, 32]. Mostly similar results were also reported from the CANVAS (Canagliflozin cardiovascular assessment study) and CANVAS-R (CANVAS-Renal) studies using canagliflozin [33, 34].
Diabetic patients are more likely to develop a rise in intraglomerular pressure resulting in glomerular hyperfiltration. This is because the function of the afferent artery is reduced, and the ability to maintain the proper intraglomerular pressure weakens [35]. Glomerular hyperfiltration is a predictor of subsequent renal dysfunction, and untreated patients have a higher frequency of proteinuria than treated patients with less hyperfiltration [36, 37]. Empagliflozin reduces intraglomerular pressure in type 1 diabetic patients and corrects glomerular hyperfiltration [38, 39]. An SGLT-2 inhibitor suppresses sodium reabsorption in the proximal tubule, and sodium chloride is transported to the macula densa. Due to the increases in sodium and chloride ions in the lumen, tubuloglomerular feedback causes the afferent artery to contract and the intraglomerular pressure to decrease [40]. SGLT-2 inhibitors are thought to be so-called regulators of intraglomerular pressure, which are capable of directly adjusting the intraglomerular pressure. The beneficial effects shown in the EMPA-REG OUTCOME trial and the CANVAS/CANVAS-R trial were attributed to appropriate control and maintenance of intraglomerular pressure by the SGLT-2 inhibitor.
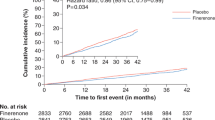
Figure 1 shows the change in eGFR in the active and placebo groups in the EMPA-REG OUTCOME trial [31]. Empagliflozin caused a temporary 5–6% decrease in eGFR after 4 weeks of treatment, but eGFR recovered after 12 weeks and stabilized at a lower value than before treatment. The treatment maintained the level as it is. As a result, a marked renal and cardiovascular protective effect was shown. It is thought that the glomerular hyperfiltration correction effect in the early stage of treatment is linked to the long-term renal protective effect. With the temporary correction of the intraglomerular pressure, the self-regulatory function of the kidney may have begun to work. Glomerular hyperfiltration cannot be judged only by the absolute value of eGFR. Even if the absolute value of eGFR is not high, it may be relatively high for the individual because of age, sex, and underlying disease. From that point of view, diabetes patients have a fairly high rate of glomerular hyperfiltration [41]. Therefore, the temporary lowering of eGFR at the initial phase is thought to be followed by a long-term protective effect against the progression of renal dysfunction, even if the absolute value of eGFR before treatment is not very high.
Change in the estimated glomerular filtration rate (eGFR) over 192 weeks in the active (empagliflozin at a dose of 10 mg or 25 mg) and placebo groups in the EMPA-REG OUTCOME trial (Ref. [32]). Baseline values are means, and the I bars indicate standard errors. The eGFR was calculated according to the creatinine formula developed by the Chronic Kidney Disease Epidemiology Collaboration
The shape of the graph of the eGFR change in the active drug group in Fig. 1 may be a new predictor of the long-term renal and cardiovascular protective effect in CKD patients. More importantly, in the EMPA-REG OUTCOME trial and the CANVAS/CANVAS-R trial, the prescription rate of ACE inhibitors and ARBs was as high as 80–84% [33, 42]. An SGLT-2 inhibitor was added to further adjust the intraglomerular pressure to obtain a beneficial clinical effect. That is, treatment with the ACE inhibitor or ARB alone was insufficient. Therefore, MR antagonists, which are another class of drugs that can adjust the intraglomerular pressure, are expected to have a renoprotective effect.
Effect of MR antagonist treatment on eGFR
Glomerular hyperfiltration has been reported to occur with an aldosterone excess [43]. Glomerular hyperfiltration with aldosterone excess has also been shown to lead to a rapid drop in eGFR when left to stand [44]. In fact, in patients with primary aldosteronism, plasma aldosterone levels have been reported to be important predictors of renal dysfunction [45]. In studies involving the general population, the aldosterone-renin ratio and plasma aldosterone concentrations were inversely correlated with the eGFR, and as plasma aldosterone concentrations increase, the risk of developing CKD increases [46]. That is, a high plasma aldosterone concentration even within the normal range suggests that glomerular pressure may be increased and may be involved in renal dysfunction. Basic experiments have demonstrated that aldosterone contracts both the afferent and efferent arteries in a dose-dependent manner, but the efferent artery contracts more strongly [47]; thus, the intraglomerular pressure rises. Therefore, glomerular capillary pressure is increased in the experimental model of mineralocorticoid-salt hypertension [48]. However, according to Arima et al., contraction of the afferent and efferent arteries by aldosterone is a nongenomic effect that is not suppressed by spironolactone and is not mediated by the classical MR [47]. There are some basic research reports on so-called putative membrane MR-mediated actions that cannot be blocked by MR antagonists. However, it has recently become clear that almost all actions of aldosterone are mediated by the classical MR [49], and in fact, data strongly suggest that the contractile actions of the efferent artery caused by aldosterone are blocked by an MR antagonist.
Administration of an MR antagonist reduces eGFR, and it is believed that relative glomerular hyperfiltration caused by aldosterone has been relieved, but what does this mean clinically for CKD treatment? Six months after surgery for an aldosterone-producing adenoma, eGFR decreased by ~15%, but urinary albumin excretion and markers for tubular damage were significantly reduced [43]. Treatment with the MR antagonist spironolactone in patients with primary aldosteronism resulted in an average 8 ml/min decrease in eGFR after 1 month, which was greater than that of the conventional treatment group. However, proteinuria was significantly reduced compared with the conventional therapy group, and the decrease in eGFR was less than that in the conventional therapy group after 1 year [50]. After surgery or medical treatment of primary aldosteronism, the decline in eGFR for up to 6 months was larger than in patients with essential hypertension, but the progression of eGFR did not differ thereafter between primary aldosteronism and essential hypertension. There were significantly more primary aldosteronism patients who improved from microalbuminuria to normoalbuminuria than essential hypertension patients [51].
The effects of an MR antagonist on eGFR have also been shown for conditions that do not necessarily cause aldosterone excess. Aldosterone breakthrough occurs in some patients as a reincrease in plasma aldosterone after some length of treatment with an ACE inhibitor or ARB [13, 52]. When aldosterone breakthrough occurs, the decrease in eGFR is enhanced [53]. The EVALUATE (The Eplerenone Combination Versus Conventional Agent to Lower Blood Pressure on Urinary Antialbuminuric Treatment Effect) trial was conducted to examine the antialbuminuric effects of the MR antagonist eplerenone in nondiabetic Japanese CKD patients with hypertension [54]. In that trial, a significant decrease in eGFR was observed 8 weeks after treatment with eplerenone, but it remained stable. The urinary albumin excretion rate in the early morning after 52 weeks, which was the primary endpoint, was significantly reduced in the eplerenone group.
From these studies, it can be seen that relative glomerular hyperfiltration is rapidly corrected with MR antagonist treatment, even without aldosterone excess. This is reflected in the initial temporary decrease in the eGFR. As a result, there are reductions in proteinuria and albuminuria in the short term. However, there are few relatively long-term, large-scale clinical studies of MR antagonists that assessed long-term prognosis and cardiovascular system outcomes in CKD patients.
Clinical significance of eGFR reduction by MR antagonists demonstrated by large-scale clinical studies targeting heart failure
The effects of MR antagonists on eGFR can be inferred from large-scale clinical trials investigating the effects of MR antagonists in patients with chronic heart failure and left ventricular dysfunction after myocardial infarction [14,15,16]. Despite the beneficial effects of MR antagonists reported by clinical studies, MR antagonists are not frequently used in conditions for which aggressive use is recommended, such as chronic heart failure with reduced systolic function (HFrEF) [55]. This is likely due to concerns about renal dysfunction and hyperkalemia caused by MR antagonists. However, when we analyze those studies in detail, completely different interpretations appear. Eplerenone has been shown to significantly reduce the composite primary endpoint of all-cause mortality, cardiovascular death, and sudden cardiac death in the EPHESUS (Eplerenone Post-acute Myocardial Infarction Heart Failure Efficacy and Survival) study for patients with left ventricular dysfunction after myocardial infarction [15]. In the subanalysis of EPHESUS, when the effect on eGFR of eplerenone was examined, there was a rapid decrease in eGFR one month after the initiation of treatment, but it was stable thereafter. One month later, patients with a 20% or greater reduction in eGFR had a higher incidence of cardiovascular events regardless of the pretreatment eGFR value if they were not taking eplerenone. However, even with a reduction of 20% or more, survival was improved, and cardiovascular events were significantly suppressed in the patients treated with eplerenone [56].
It has been shown in EMPHASIS-HF (the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure) study, which examined the effects of eplerenone in patients with mild HFrEF, that the combined primary endpoint of cardiovascular death and first hospitalization for heart failure was significantly reduced.
Total mortality was also reduced, and life expectancy was improved [16]. In the subanalysis, after 21 months of treatment, in 27% of cases, the eGFR decreased by 20% or more, and in 14% of cases, it decreased by 30% or more. Although this rate was high in the eplerenone treatment group, there was a significant decrease in total mortality in the eplerenone group. The eGFR in the eplerenone group dropped rapidly in the first 5 months, which was consistent with the behavior seen in previous reports [57]. After MR antagonist treatment, eGFR decreases temporarily, and it appears that renal function has deteriorated. However, even if renal function has actually deteriorated, there is evidence for a reduction in all-cause and cardiovascular death. That is, the initial transient decrease in eGFR by the MR antagonist appears to work effectively to provide fine adjustment of glomerular pressure, and this works advantageously to suppress long-term cardiovascular events. Subgroup analysis of the groups with eGFR under 60 ml/min also showed the clinical efficacy of an MR antagonist, since it did not inherently impair renal function [58]. Such an effect is likely to apply to the renal protection of CKD treatment. If glomerular pressure can be controlled appropriately, it is thought that long-term renal protective effects will occur. If the change in eGFR similar to that shown in Fig. 1 can be confirmed after the treatment with MR antagonist, it can be inferred that the intraglomerular pressure is properly controlled.
At what stage of CKD can the clinical effects of MR antagonism be expected? The majority of previous clinical studies have shown that an MR antagonist is most effective in patients who have early stages of CKD with maintained eGFR. The BARACK D (benefit of aldosterone receptor antagonism in chronic kidney disease) trial is currently underway to investigate the effects of spironolactone in patients with CKD stage G3b [59]. The group with 25 mg of spironolactone added to the standard treatment will be compared with the placebo group, and the primary endpoint will not be a surrogate marker, such as albuminuria or BNP, but death or a cardiovascular event, and an impact will be obtained if the result is superior.
CKD treatment from now on considering intraglomerular pressure adjustment
Figure 2 shows the main hormones and peptides that act on the renal afferent and efferent arteries and directly alter intraglomerular pressure. Among them, ACE inhibitors and ARBs are thought to act on the efferent artery, and SGLT-2 inhibitors are thought to act on the afferent artery. An MR antagonist acts relatively more on the efferent artery [47]. ACE inhibitors and ARBs are the main therapeutic agents for CKD, but it is difficult to sufficiently delay CKD progression. Therefore, an SGLT-2 inhibitor has been added to provide further renal protection and suppress cardiovascular events [30, 33, 34]. If there is an additional MR antagonist, will there be further beneficial effects?
The main hormones and peptides that act on the renal afferent and efferent arteries and directly alter intraglomerular pressure. NO nitric oxide, COX-2 cyclooxygenase-2, ANP atrial natriuretic peptide, Ang 1–7 angiotensin 1–7 (one to seven), Ang II angiotensin II; TXA2, thromboxane A2, ET-1 endothelin-1, ROS reactive oxygen species, ALDO aldosterone
The CVD-REAL Nordic trial comparing cardiovascular events of type 2 diabetic patients who started an SGLT-2 inhibitor or other hypoglycemic agent has been helpful [60]. In that trial, an ACE inhibitor or ARB was prescribed to ~67%, but the MR antagonist prescription rate was very low, at 4.4%. In the group prescribed an MR antagonist, the incidences of fatal cardiovascular events and major cardiovascular events were reduced.
Conclusion
Relative glomerular hyperfiltration is rapidly corrected with MR antagonist treatment, even without aldosterone excess. This is reflected in the initial temporary decrease in the eGFR. As a result, there are reductions in proteinuria and albuminuria in the short term. A frequent temporary decrease in eGFR at the initial phase and significant reductions in total mortality and cardiovascular events by MR antagonist have been observed in patients with chronic heart failure. It is expected that a number of relatively long-term, large-scale clinical research trials targeting renal events and all-cause and cardiovascular death in CKD patients treated with an MR antagonist will be planned.
References
Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United State. JAMA. 2007;298:2038–47.
Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–15.
Kottgen A, Russell SD, loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307–15.
Manjunath G, Tighiouart H, Ibrahim H, MacLeod B, Salem DN, Griffith JL, et al. Level of kidney function as a risk factor for atherosclerosis cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55.
Cheng TY, Wen SF, Astor BC, Tao XG, Samet JM, Wen CP. Mortality risks for all causes and cardiovascular diseases and reduced eGFR in a middle-aged working population in Taiwan. Am J Kidney Dis. 2008;52:1051–60.
Sato A. The necessity and effectiveness of mineralocorticoid receptor antagonist in the treatment of diabetic nephropathy. Hyertens Res. 2015;38:367–74.
Shibata S, Ishizawa K, Uchida S. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens Res. 2017;40:221–5.
Nishimoto M, Ohtsu H, Marumo T, Kawarazaki W, Ayuzawa N, Ueda K, et al. Mineralocortiocid receptor blockade suppresses dietary salt-induced ACEI/ARB-resistant albuminuria in non-diabetic hypertension: a sub-analysis of evaluate study. Hypertens Res. 2019;42:514–21.
Chrysostomou A, Becker G. Spironolactone in addition to ACE inhibition to reduce proteinuria in patients with chronic renal failure. N Engl J Med. 2001;345:925–6.
Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Rossing P, Tarnow L, et al. Beneficial impact of spironolactone in diabetic nephropathy. Kidney Int. 2005;68:2829–36.
Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Tarnow L, Rossing P, et al. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2006;70:536–42.
Rachmani R, Slavachevsky I, Amit M, Levi Z, Kedar Y, Berla M, et al. The effect of spironolactone, cilazapril and their combination on albuminuria in patients with hypertension and diabetic nephropathy is independentof blood pressure reduction: a randomized controlled study. Diabet Med. 2004;21:471–5.
Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41:64–8.
Pitt B, Zannad F, Remme W, Cody R, Castaigne A, Perez A. et al. for The Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17.
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. for The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21.
Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. for the EMPHASIS-HF Study Group. Eplrerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21.
Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–8.
Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J. Change inestimated GFR association with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20:2617–24.
Barzilay JI, Davis B, Pressel SL, Ghosh A, Rahman M, Einhorn PT, et al. The effects of eGFR change on CVD, renal, and mortality outcomes in a hypertensive cohoert treated with 3 different antihypertensive medications. Am J Hypertens. 2018;31:609–14.
Al-Aly Z, Zeringue A, Fu J, Rauchman MI, McDonald JR, El-Achkar TM, et al. Rate of kidney function decline associates with mortality. J Am Soc Nephrol. 2010;21:1961–9.
Clase CM, Barzillay J, Gao P, Smyth A, Schmieder RE, Tobe S, et al. Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcome. Kidney Int. 2017;91:683–90.
Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJL, et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease on long-term renal function. Kidney Int. 2011;80:282–7.
Apperloo AJ, de Zeeuw D, de Jong PE. A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int. 1997;51:793–7.
Hansen HP, Rossing P, Tarnow L, Nielsen FS, Jensen BR, Parving HH. Increased glomerular filtration rate after withdrawal of long-term antihypertensive treatment in diabetic nephropathy. Kidney Int. 1995;47:1726–31.
Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-asociated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–93.
Wright JT Jr., Bakris G, Green T, Agodoa LY, Appel LJ, Charleston J, et al. African American Study of Kidney Disease and Hypertension Study Group: Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the ASSK trial. JAMA. 2002;288:2421–31.
Collard D, Brouwer TF, Oeters RJG, Vogt L, van den Born BJH. Creatinine rise during blood pressure therapy and the risk of adverse clinical outcomes in patients with type 2 diabetes mellitus. A post hoc analysis of the ACCORD-BP randomized controlled trial. Hypertension. 2018;72:1337–44.
Ku E, Bakris G, Johansen KL, Lin F, Sarnak MJ, Campese VM, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol. 2017;28:2794–801.
Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, et al. for the SPRINT Research Group. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812–23.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. for the EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Cherney DZI, Zinman B, Inzucchi S, Koitka-Weber A, Mattheus M, von Eynatten M, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from EMPA-REG OUTCOME randomized, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–21.
Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomized clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704.
Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, et al. Cariovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138:1537–50.
Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, et al. on behalf of the EMPA-REG OUTCOME investigators. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137:119–29.
Ruggenenti P, Porrini EL, Gaspari F, Motterlini N, Cannata A, Carrara F, et al. for the GFR Study Investigators. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012;35:2061–8.
Anderson S, Brenner BM. Intraglomerular hypertension: implications and drug treatment. Annu Rev Med. 1988;39:243–53.
Strtic M, Yang GK, Perkins BA, Soleymanlou N, Lytvyn Y, von Eynatten M, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia. 2014;57:2599–602.
Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, et al. Renal hemodynamics effect of sodium-glucose cotransporter-2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–97.
Mima A. Renal protection by sodium-glucose cotransporter 2 inhibitors and its underlying mechanisms in diabetic kidney disease. J Diabetes Complicat. 2018;32:720–5.
Jerums G, Premaratne E, Panagiotopoulos P, Maclsaac RJ. The clinical significance of hyperfiltration in diabetes. Diabetologia. 2010;53:2093–2014.
Christensen PK, Hansen HP, Parving HH. Impaired autoregulation of GFR in hypertensive non-insulin dependent diabetic patients. Kidney Int. 1997;52:1369–74.
Ribstein J, Du Cailar G, Fesler P, Mimran A. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol. 2005;16:1320–5.
Rossi GP, Bermini G, Desideri G, et al. PAPY Study Participants. Renal damage in primary aldosteronism; results of the PAPY study. Hypertension. 2006;48:232–8.
Reincke M, Rump LC, Quinkler M, Hahner S, Diederich S, Lorenz R, et al. Risk factors associated with a low glomerular filtration rate in primary aldosteronism. J Clin Endocrinol Metab. 2009;94:869–75.
Hannemann A, Rettig R, Dittmann K, Volzke H, Endlich K, Nauck M, et al. Aldosterone and glomerular filtration-observations in the general population. BMC Nephrol. 2014;15:44.
Arima S, Kohaguro K, Xu HL, Sugawara A, Abe T, Satoh F, et al. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J Am Soc Nephrol. 2003;14:2255–63.
Dworkin LD, Hosrerrer TH, Rennke HG, Brenner BM. Hemodynamic basis for glomerular injury in rats with deoxycorticosterone-salt hypertension. J Clin Invest. 1984;73:1448–61.
Funder JW. Aldosterone and mineralocorticoid receptors: A personal reflection. Mol Cell Endcrinol. 2012;350:146–50.
Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70:2116–23.
Sechi LA, Novello M, Lapenna R, Baroselli S, Nadalini E, Colussi GL, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295:2635–45.
Sato A, Saruta T. Aldosterone breakthrough during angiotensin-converting enzyme inhibitor therapy. Am J Hypertens. 2003;16:781–8.
Schjoedk KJ, Andersen S, Rossing P, Tarnow L, Parving HH. Aldosterone escape during blockade of renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia. 2004;47:1936–9.
Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T, for the EVALUATE Study Group. Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: a double-blind, randomized, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:944–53.
Albert NM, Yancy CW, Liant L, Zhao X, Hernandez AF, Peterson ED, et al. Use of aldosterone antagonists in heart failure. JAMA. 2009;302:1658–65.
Rossignol P, Cleland JG, Bhandari S, Tala S, Gustafsson F, Fay R, et al. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: insights from the Eplerenone post-acute myocardial infarction heart failure effeicacy and survival study. Circulation. 2012;125:271–9.
Rossignol P, Dobre D, McMurray JJV, Swedberg K, Krum H, van Veldhuisen DJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy. Results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail. 2014;7:51–8.
Eschalier R, McMurray JJV, Swedberg K, van Veldhuisen DJ, Krum H, Pocock SJ, et al. for the EMPHASIS-HF Investigators. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function. J Am Coll Cardiol. 2013;62:1585–93.
Hill NR, Lasserson D, Thompson B, Perera-Salazar R, Wolstenholme J, Bower P, et al. Benefits of aldosterone receptor antagonism in chronic kidney disease (BARACK D) trial-a multi-centre, prospective, randomized, open, blinded end-point, 36-month study of 2,616 patients with primary care with stage 3b chronic kidney disease to compare the efficacy of spironolactone 25 mg once daily in addition to routine care on mortality and cardiovascular outcomes versus routine care alone: study protocol for a randomized controlled trial. Trials. 2014;15:160.
Birkeland KI, Jørgensen ME, Carstensen B, Persson F, Gulseth HL, Thuresson M, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol 2017;5:709–17.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that he has no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sato, A. Does the temporary decrease in the estimated glomerular filtration rate (eGFR) after initiation of mineralocorticoid receptor (MR) antagonist treatment lead to a long-term renal protective effect?. Hypertens Res 42, 1841–1847 (2019). https://doi.org/10.1038/s41440-019-0320-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0320-9
Keywords
This article is cited by
-
Antihypertensive effects and safety of esaxerenone in patients with moderate kidney dysfunction
Hypertension Research (2021)
-
Apararenone in patients with diabetic nephropathy: results of a randomized, double-blind, placebo-controlled phase 2 dose–response study and open-label extension study
Clinical and Experimental Nephrology (2021)