Abstract
Inappropriate sympathetic activation is closely associated with the development and progression of hypertension. Renal denervation (RDN) is a neuromodulation therapy performed using an intraarterial catheter in patients with hypertension. Recent randomized sham-operated controlled trials have shown that RDN has significant antihypertensive effects that last for at least 3 years. Based on this evidence, RDN is nearly ready for general clinical application. On the other hand, there are remaining issues to be addressed, including elucidation of the precise antihypertensive mechanisms of RDN, the appropriate endpoint of RDN during the procedure, and the association between reinnervation after RDN and the long-term effects of RDN. This mini review focuses on studies implicating anatomy of the renal nerves, which consist of afferent or efferent and sympathetic or parasympathetic nerves, the response of blood pressure to renal nerve stimulation, and reinnervation of renal nerves after RDN. A comprehensive understanding of the anatomical and functional aspects of the renal nerves and the antihypertensive mechanisms of RDN, including its long-term effects, will enhance our ability to incorporate RDN into strategies to treat hypertension in clinical practice.

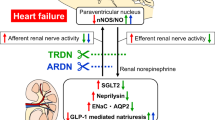
This mini review focuses on studies implicating anatomy of the renal nerves, which consist of afferent or efferent and sympathetic or parasympathetic nerves, the response of blood pressure to renal nerve stimulation, and reinnervation of renal nerves after renal denervation. Whether the ablation site is sympathetic dominant or parasympathetic dominant, and afferent dominant or efferent dominant, would in turn determine the final output of renal denervation. BP: blood pressure.
Similar content being viewed by others
Introduction
In patients with essential hypertension, there is increased spillover of norepinephrine in the whole body, the heart and the kidney as well as augmented muscle sympathetic nerve activity [1]. Renal denervation (RDN) has been developed as a neuromodulation therapy for patients with hypertension [2]. Denervation techniques include radiofrequency ablation, ultrasound ablation, and chemical ablation using alcohol, all of which involve transcatheter denervation from the lumen of the renal artery. The early SYMPLICITY HTN-3 trial, which was the first large, blinded, randomized sham-operated controlled trial on uncontrolled treatment-resistant hypertension, did not show a statistically significant effect of RDN at 6 months of follow-up [3], possibly due to numerous confounding factors. Since then, optimization of the study design—including the procedural techniques, development of new devices, standardization of medication, assessment of medication adherence, exclusion of hyperaldosteronism, and selected primary endpoints—has been reconsidered [4,5,6,7]. Consequently, recent randomized sham-operated controlled trials (i.e., the SPYRAL HTN-OFF MED/ON MED trial and RADIANCE-HTN SOLO/TRIO trial) have shown that RDN has a clear antihypertensive effect [8,9,10,11]. Ogoyama et al. reported a meta-analysis of nine randomized sham-controlled trials of RDN that suggested an established effect of RDN on office, home and 24 h blood pressure (BP) parameters in patients with resistant, uncontrolled, and drug-naïve hypertension [12]. Two other studies reported that the antihypertensive effects of RDN had persisted for at least 3 years [13, 14]. This evidence suggests that RDN is near to general clinical application. However, the precise mechanisms underlying the antihypertensive effect of RDN have not yet been fully elucidated. In addition, several issues pertaining to clinical application remain to be resolved, including the determination of therapeutic efficacy and the setting of endpoints during the RDN procedure. To address these issues, this mini review summarizes the latest findings, focusing on renal nerve anatomy, the response of BP to renal nerve stimulation (RNS), the long-term effects of RDN, and renal nerve reinnervation after RDN.
Anatomy of the renal nerves revisited
Two interesting topics regarding the anatomy of the renal nerves have recently been investigated using genetically modified mouse models and immunohistochemistry. One is the distribution of the sensory afferent nerves in the kidney. The sensory afferent nerves are characterized by immunolabeling for the neuropeptides calcitonin gene-related peptide (CGRP) and substance P (SP), which are the dominant neurotransmitters for renal afferents, and the transient receptor potential vanilloid 1 channel, which is expressed on sensory afferent nerves and activated by capsaicin. Many anatomical studies using anterograde and retrograde tracing combined with immunohistochemistry for CGRP and SP have demonstrated that the majority of the afferent renal nerves are distributed in the renal pelvic wall, with a sparse distribution in the renal cortex [15,16,17,18,19]. However, recent investigations using a transgenic mouse model combined with immunohistochemistry under analyses by high-resolution confocal microscopy have revealed that the afferent renal nerves are distributed more substantially in the renal cortex than previously appreciated. Osborn et al. demonstrated that the afferent fibers immunoreactive to CGRP are adjacent to the glomerular podocytes immunoreactive to nephrin in the renal cortex. Based on these findings, the group hypothesized that the afferent renal nerves may interact with the sympathetic efferent nerves to regulate the glomerular filtration rate [20]. This hypothesis seems plausible because the renal nerves can convey information related with environmental changes such as the renal blood flow or glomerular filtration rate from the renal cortex to the central nervous system promptly.
The other topic is the distribution of the parasympathetic nerves in the kidney. Whether the kidney has parasympathetic innervation has been controversial [20, 21]. Some previous studies using a retrograde tracer demonstrated that the nodose ganglion contains the cell bodies of neurons that project to the kidney in rats and mice [22,23,24], suggesting the presence of vagal afferents in the kidney. However, there is no evidence showing the existence of vagal efferents in the kidney. Recently, Cheng et al. found anatomical evidence for parasympathetic innervation of the kidney [25]. This study using transgenic mice with specific expression of the Cre recombinase in choline acetyltransferase-expressing cells (Chat-ires-Cre mice [26]) and Cre reporter mice with tdTomato fluorescence (Ai14 mice [27]) combined with immunostaining for choline acetyltransferase and vesicular acetylcholine transporter, which are widely accepted specific markers for cholinergic nerves [28, 29], demonstrated that cholinergic nerves supply the renal vasculature and pelvis. There were also cholinergic ganglion cells within the renal nerve plexus. Moreover, the single-cell RNA sequencing revealed that acetylcholine receptors are expressed in the renal artery and its segmental branches. In addition, retrograde tracing using recombinant adeno-associated virus and pseudorabies virus followed by the imaging of the brain, spinal cord, and ganglia demonstrated that vagal afferents conduct the renal sensory pathway to the nucleus tractus solitarius (NTS), and vagal efferents project from the dorsal motor nucleus of the vagus nerves (DMV) to the kidney. It is interesting to note that on the renal artery, the newly discovered cholinergic nerve fibers are separated not only from the sympathetic nerves but also from the sensory nerves. Figure 1 shows the updated neural circuitry of efferent and afferent innervation in the kidney. Further studies are needed to determine their functions, particularly the roles of the sensory afferent and parasympathetic nerves in the kidney.
Renal nerve stimulation
RNS has been studied as a method to guide the choice of the optimal ablation site and to determine an endpoint for RDN during the procedure. Five different patterns of BP responses to RNS have been observed in hypertensive dogs: 1) Continuous ascending and finally keeping steady above baseline, 2) Declining and then rising over baseline, 3) Declining and then rising but below baseline, 4) Fluctuating in the vicinity of the baseline, and 5) Continuous declining and finally keeping steady below the baseline [30]. In summary, in about 40% of cases, RNS ultimately increases BP, while in another 40% of cases it causes no change of BP, and in about 20% of cases RNS ultimately decreases BP. Denervation of the renal nerves with increased BP in response to RNS is presumed to be important for lowering BP by RDN in follow-up months. In fact, Liu et al. reported that selective ablation of sites with maximum systolic BP elevation >10 mmHg during RNS in hypertensive dogs resulted in greater antihypertensive effects than ablation of sites with systolic BP elevation ≤10 mmHg or depressor sites [31]. In an extension of this study, the same research group reported that a selective RDN consisting of ablation at sites with systolic BP elevation >10 mmHg during RNS had a greater renal norepinephrine content reduction and greater suppression of the intrarenal renin-angiotensin system and transporter expression involved in sodium and water reabsorption [32]. A disappearance of the RNS-induced BP elevation immediately after RDN during the procedure was also correlated with the subsequent antihypertensive effect of RDN [33]. It is plausible that the sympathoexcitatory renal afferent reflex mediated the increase in BP in response to RNS. However, which nerve stimulation produces which BP response among the afferent or efferent nerve and sympathetic or parasympathetic nerve remains to be fully elucidated. Recently, an RNS trial was conducted in a clinical setting [34]. Forty-four treatment-resistant hypertensive patients were included in the prospective, single-center RNS trial. Before RDN, the RNS-induced systolic BP rise was 43 mmHg, and decreased to 9 mmHg immediately after RDN. Mean 24-h systolic BP decreased from 147 mmHg at baseline to 135 mmHg at a 10-month follow-up. The RNS-induced BP changes before RDN were correlated with 24-h systolic BP changes at follow-up. Patients with ≤0 mmHg residual RNS-induced BP response after RDN had a significantly lower mean 24-h systolic BP at follow-up compared to the patients with >0 mmHg residual RNS-induced BP response (126 ± 4 mmHg versus 135 ± 10 mmHg, p < 0.05). Most of the patients (83%) with ≤0 mmHg residual RNS-induced BP response had normal 24-h systolic BP at follow-up, whereas a significant proportion of patients (67%) with a residual RNS-induced BP response remained hypertensive at follow-up. These findings underline the idea that RNS can be useful to validate the endpoint of successful denervation during the procedure.
Long-term efficacy of renal denervation on hypertension
One of the unsolved issues in regard to RDN is its long-term efficacy on hypertension. Recently, several sham-operated randomized controlled trials have reported the long-term efficacy of RDN. The SYMPLICITY HTN-3 trial using the first generation Symplicity Flex catheter did not show antihypertensive efficacy of RDN at a 6-month follow-up [3]. However, recent analysis of long-term data from the SYMPLICITY HTN-3 trial showed that patients in the RDN group had significantly larger reductions from baseline to 36 months of follow-up in both office and 24 h ambulatory systolic BP compared with the sham control group [13]. The study included patients with treatment-resistant hypertension who were taking maximally tolerated doses of three or more antihypertensive drugs including a diuretic and still had an office systolic BP of 160 mmHg or more and a 24-h ambulatory systolic BP of 135 mmHg or more. The short-term analysis at 6 months of follow-up did not show a significant difference of BP reduction between an RDN group and sham group due to the major placebo effect observed in the sham group. However, the long-term analysis at 12 to 36 months showed that RDN had a significant efficacy, and that the longer the treatment term, the stronger the antihypertensive effect. Recent sham-operated control trials using second generation catheters, such as SPYRAL HTN-OFF MED/ON MED trials and RADIANCE-HTN SOLO/TRIO trials, established the short-term efficacy of RDN at 2 to 6 months [12]. Three-year long-term results have been reported for the SPYRAL HTN-ON MED trial. The results showed that the efficacy of RDN was not only maintained for 3 years, but also enhanced over time [14]. Moreover, Kario et al. subsequently reported that morning (7:00–9:00AM) and nighttime (1:00–6:00AM) ambulatory systolic BP in SPYRAL HTN ON-MED patients at 36 months were significantly lower for the RDN group compared to sham controls [35], suggesting the beneficial effect of RDN on reducing the incidence of cardiovascular events associated with morning and nighttime hypertension.
Reinnervation of renal nerves after renal denervation
Whether reinnervation occurs after RDN has been a subject of active debate. The results of histological evaluation by immunostaining for tyrosine hydroxylase (TH), neuropeptide Y (NPY), CGRP, and SP and measurement of renal tissue norepinephrine (NE) and CGRP content have been mixed. Table 1 provides a summary of animal studies investigating reinnervation of the renal nerves after RDN. In normotensive rats, unilateral surgical RDN decreased TH, NPY, CGRP and SP labeling to 10% or less of that in the contralateral innervated kidney within 1 week. Labeling with these markers returned to baseline within 12 weeks. Renal tissue NE and CGRP content decreased by 78% and by 72%, respectively, at 1 week after RDN. The tissue NE levels did not recover, while CGRP levels almost fully recovered at 12 weeks after RDN [36, 37]. In Lewis Polycystic Kidney rats, TH labelling was significantly reduced to 4% of sham levels and CGRP labelling was reduced to 8% of sham levels following bilateral surgical RDN at 1 week. At 4 weeks after RDN, TH and CGRP labeling recovered to 65% and 42% of the sham levels, respectively [38]. In spontaneously hypertensive rats (SHR), bilateral surgical RDN reduced renal NE content to 13%, 25%, 33% and 35% of that found in sham-operated SHR at 2, 4, 6 and 8 weeks, respectively, after RDN [39]. In normotensive sheep, one week after radiofrequency-based intraarterial RDN by using a Symplicity Flex catheter, the levels of renal cortical TH and pelvic CGRP staining were significantly lower compared with those in the nondenervated group. Both TH and CGRP staining were partially recovered at 5.5 months and fully recovered at 11 months after RDN. Renal cortical NE content was decreased to 20.1% of the control level at one week after RDN. The mean cortical levels of NE were 88.9% and 131.0% at 5.5 months and 11 months, respectively, after RDN [40]. In this study, RNS caused no changes in BP immediately after RDN. However, RNS increased BP to normal levels at 5.5 and 11 months after RDN [40]. In sheep with hypertensive chronic kidney disease (CKD), 30 months after radiofrequency RDN by a Symplicity Flex catheter, the levels of TH and CGRP staining in the kidneys were significantly lower by 50% and by 67%, respectively, compared with those in sham-operated CKD sheep. Renal NE content was decreased by 49% and vasoconstriction in response to RNS was also decreased by 56% compared with sham-operated CKD sheep. These results suggest that anatomical and functional reinnervation of the renal nerves occurred 30 months after RDN, but the levels were only partially restored to the levels of intact [41]. In normotensive swine, attenuation of TH staining in the kidneys induced by radiofrequency RDN using a Symplicity Flex catheter peaked at 7 days, was sustained until 30 days, and partially recovered at 60 and 180 days [42]. Another study using healthy swine reported that radiofrequency RDN using an Iberis catheter strongly decreased TH staining in the renal nerves at 7 days. At 90 days after RDN, some TH expression with disorganized architecture was observed in the renal nerves, suggesting that the regenerative activity is unlikely to restore function [43]. The renal NE content of normotensive swine was significantly reduced after radiofrequency RDN with an Iberis catheter. However, the degree of the reduction of renal NE concentration was less at 90 days than at 7 and 30 days [44]. A more recent study by Sharp et al. using normotensive swine treated with radiofrequency RDN using a Spyral catheter demonstrated that renal cortical axon density assessed by quantitative TH staining and corresponding cortical NE levels were significantly reduced in the RDN versus the control group at 7 days and remained suppressed through 28 and 180 days [45]. These results were consistent with the long-term effect of RDN reported in recent clinical studies.
A summary of the results of previous studies on reinnervation after RDN suggests the following. (1) Anatomic reinnervation does not necessarily mean functional reinnervation. (2) There may be a compensatory increase in the function of partially damaged or undamaged residual nerves. (3) The effects of RDN and the process of reinnervation may differ between physiological and pathological conditions. Further investigations to address these issues are needed.
Future perspectives
Recently, Biffi et al. reported a meta-analysis showing that RDN significantly reduced muscle sympathetic nerve activity accompanied by a significant BP decrease, suggesting that the antihypertensive effect of RDN might be mediated, in part, by sympathoinhibition, possibly via a central mechanism [46]. The antihypertensive effect of RDN is probably dependent on the ablation target site in the renal nerves. Whether the ablation site is sympathetic dominant or parasympathetic dominant, and afferent dominant or efferent dominant, would in turn determine the final output of RDN, such as the sympathoinhibitory effect or sympathoexcitatory effect, or the depressor effect or pressor effect of RDN. Future research should be directed toward further clarification of the functions of each renal nerve population, including their differences between physiological and pathological conditions, to create more effective RDN strategies.
References
Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43:169–75.
Katsurada K, Shinohara K, Aoki J, Nanto S, Kario K. Renal denervation: basic and clinical evidence. Hypertens Res. 2022;45:198–209.
Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N. Engl J Med. 2014;370:1393–401.
Weber MA, Mahfoud F, Schmieder RE, Kandzari DE, Tsioufis KP, Townsend RR, et al. Renal denervation for treating hypertension: current scientific and clinical evidence. JACC Cardiovasc Inter. 2019;12:1095–105.
Kandzari DE, Mahfoud F, Bhatt DL, Bohm M, Weber MA, Townsend RR, et al. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension. 2020;76:1410–7.
Kario K, Kim BK, Aoki J, Wong AY, Lee YH, Wongpraparut N, et al. Renal Denervation in Asia: consensus statement of the Asia Renal Denervation Consortium. Hypertension. 2020;75:590–602.
Mogi M, Maruhashi T, Higashi Y, Masuda T, Nagata D, Nagai M, et al. Update on hypertension research in 2021. Hypertens Res. 2022;45:1276–97.
Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–51.
Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–55.
Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–45.
Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397:2476–86.
Ogoyama Y, Tada K, Abe M, Nanto S, Shibata H, Mukoyama M, et al. Effects of renal denervation on blood pressures in patients with hypertension: a systematic review and meta-analysis of randomized sham-controlled trials. Hypertens Res. 2022;45:210–20.
Bhatt DL, Vaduganathan M, Kandzari DE, Leon MB, Rocha-Singh K, Townsend RR, et al. Long-term outcomes after catheter-based renal artery denervation for resistant hypertension: final follow-up of the randomised SYMPLICITY HTN-3 Trial. Lancet. 2022;400:1405–16.
Mahfoud F, Kandzari DE, Kario K, Townsend RR, Weber MA, Schmieder RE, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet. 2022;399:1401–10.
Osborn JW, Foss JD. Renal Nerves and Long-Term Control of Arterial Pressure. Compr Physiol. 2017;7:263–320.
Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 2015;308:R79–95.
Kopp UC, Cicha MZ, Smith LA, Hokfelt T. Nitric oxide modulates renal sensory nerve fibers by mechanisms related to substance P receptor activation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R279–290.
Ferguson M, Bell C. Ultrastructural localization and characterization of sensory nerves in the rat kidney. J Comp Neurol. 1988;274:9–16.
Marfurt CF, Echtenkamp SF. Sensory innervation of the rat kidney and ureter as revealed by the anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) from dorsal root ganglia. J Comp Neurol. 1991;311:389–404.
Osborn JW, Tyshynsky R, Vulchanova L. Function of Renal Nerves in Kidney Physiology and Pathophysiology. Annu Rev Physiol. 2021;83:429–50.
Okusa MD, Rosin DL, Tracey KJ. Targeting neural reflex circuits in immunity to treat kidney disease. Nat Rev Nephrol. 2017;13:669–80.
Norvell JE, Anderson JM. Assessment of possible parasympathetic innervation of the kidney. J Auton Nerv Syst. 1983;8:291–4.
Gattone VH 2nd, Marfurt CF, Dallie S. Extrinsic innervation of the rat kidney: a retrograde tracing study. Am J Physiol. 1986;250:F189–196.
Ong J, Kinsman BJ, Sved AF, Rush BM, Tan RJ, Carattino MD, et al. Renal sensory nerves increase sympathetic nerve activity and blood pressure in 2-kidney 1-clip hypertensive mice. J Neurophysiol. 2019;122:358–67.
Cheng X, Zhang Y, Chen R, Qian S, Lv H, Liu X, et al. Anatomical evidence for parasympathetic innervation of the renal vasculature and pelvis. J Am Soc Nephrol. 2022;33:2194–210.
Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204.
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–40.
Wu D, Hersh LB. Choline acetyltransferase: celebrating its fiftieth year. J Neurochem. 1994;62:1653–63.
Schafer MK, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for the vesicular acetylcholine transporter. II. The peripheral nervous system. Neuroscience. 1998;84:361–76.
Zhou H, Li Y, Xu Y, Liu H, Lai Y, Tan K, et al. Mapping renal innervations by renal nerve stimulation and characterizations of blood pressure response patterns. J Cardiovasc Transl Res. 2022;15:29–37.
Liu H, Chen W, Lai Y, Du H, Wang Z, Xu Y, et al. Selective renal denervation guided by renal nerve stimulation in canine. Hypertension. 2019;74:536–45.
Lai Y, Zhou H, Chen W, Liu H, Liu G, Xu Y, et al. The intrarenal blood pressure modulation system is differentially altered after renal denervation guided by different intensities of blood pressure responses. Hypertens Res. 2023;46:456–67.
Huang HC, Cheng HM, Chia YC, Li Y, Van Minh H, Siddique S, et al. The role of renal nerve stimulation in percutaneous renal denervation for hypertension: A mini-review. J Clin Hypertens. 2022;24:1187–93.
Hoogerwaard AF, Adiyaman A, de Jong MR, Smit JJ, Heeg JE, van Hasselt B, et al. Renal nerve stimulation: complete versus incomplete renal sympathetic denervation. Blood Press. 2021;30:376–85.
Kario K, Mahfoud F, Kandzari DE, Townsend RR, Weber MA, Schmieder RE, et al. Long-term reduction in morning and nighttime blood pressure after renal denervation: 36-month results from SPYRAL HTN-ON MED trial. Hypertens Res. 2023;46:280–8.
Mulder J, Hokfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R675–682.
Rodionova K, Fiedler C, Guenther F, Grouzmann E, Neuhuber W, Fischer MJ, et al. Complex reinnervation pattern after unilateral renal denervation in rats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R806–818.
Li S, Hildreth CM, Rahman AA, Barton SA, Wyse BF, Lim CK, et al. Renal denervation does not affect hypertension or the renin-angiotensin system in a rodent model of juvenile-onset polycystic kidney disease: clinical implications. Sci Rep. 2021;11:14286.
Kline RL, Stuart PJ, Mercer PF. Effect of renal denervation on arterial pressure and renal norepinephrine concentration in Wistar-Kyoto and spontaneously hypertensive rats. Can J Physiol Pharm. 1980;58:1384–8.
Booth LC, Nishi EE, Yao ST, Ramchandra R, Lambert GW, Schlaich MP, et al. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension. 2015;65:393–400.
Singh RR, McArdle ZM, Iudica M, Easton LK, Booth LC, May CN, et al. Sustained decrease in blood pressure and reduced anatomical and functional reinnervation of renal nerves in hypertensive sheep 30 months after catheter-based renal denervation. Hypertension. 2019;73:718–27.
Sakakura K, Tunev S, Yahagi K, O’Brien AJ, Ladich E, Kolodgie FD, et al. Comparison of histopathologic analysis following renal sympathetic denervation over multiple time points. Circ Cardiovasc Inter. 2015;8:e001813.
Rousselle SD, Brants IK, Sakaoka A, Hubbard B, Jackson ND, Wicks JR, et al. Neuromatous regeneration as a nerve response after catheter-based renal denervation therapy in a large animal model: immunohistochemical study. Circ Cardiovasc Inter. 2015;8:e002293.
Sakaoka A, Rousselle SD, Hagiwara H, Tellez A, Hubbard B, Sakakura K. Safety of catheter-based radiofrequency renal denervation on branch renal arteries in a porcine model. Catheter Cardiovasc Inter. 2019;93:494–502.
Sharp ASP, Tunev S, Schlaich M, Lee DP, Finn AV, Trudel J, et al. Histological evidence supporting the durability of successful radiofrequency renal denervation in a normotensive porcine model. J Hypertens. 2022;40:2068–75.
Biffi A, Dell’Oro R, Quarti-Trevano F, Cuspidi C, Corrao G, Mancia G, et al. Effects of renal denervation on sympathetic nerve traffic and correlates in drug-resistant and uncontrolled hypertension: a systematic review and meta-analysis. Hypertension. 2023;80:659–67.
Funding
This work was supported in part by JSPS KAKENHI Grant Number JP21K16094, MSD Life Science Foundation, Public Interest Incorporated Foundation and Jichi Medical University Young Investigator Award (to K. Katsurada).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Kario MD, PhD. received speaker fees and works as a consultant to JIMRO Co., Ltd., Medtronic Co. Inc. and Terumo Co. Inc. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katsurada, K., Kario, K. Emerging topics on renal denervation in hypertension: anatomical and functional aspects of renal nerves. Hypertens Res 46, 1462–1470 (2023). https://doi.org/10.1038/s41440-023-01266-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01266-2
Keywords
This article is cited by
-
Do tissue sodium levels support renal denervation?
Hypertension Research (2023)
-
Celiac ganglia: potential new targets in neuromodulation for hypertension
Hypertension Research (2023)